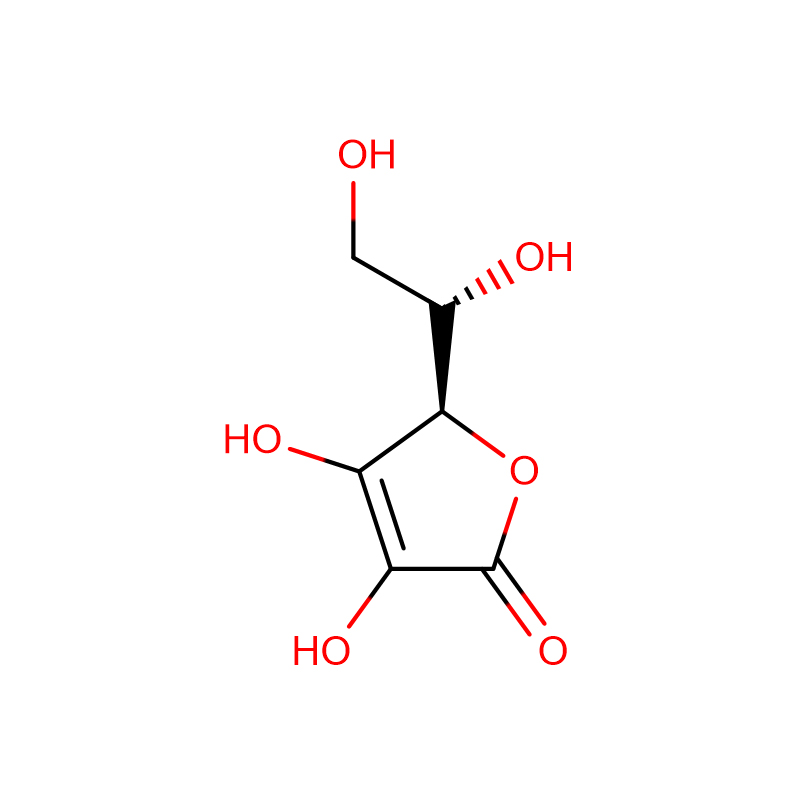
Vitamin C (Ascorbic Acid) Cas: 50-81-7
| Catalog Number | XD91869 |
| Product Name | Vitamin C (Ascorbic Acid) |
| CAS | 50-81-7 |
| Molecular Formula | C6H8O6 |
| Molecular Weight | 176.12 |
| Storage Details | 5-30°C |
| Harmonized Tariff Code | 29362700 |
Product Specification
| Appearance | White powder |
| Assay | 99% min |
| Melting point | 190-194 °C (dec.) |
| alpha | 20.5 º (c=10,H2O) |
| Boiling point | 227.71°C (rough estimate) |
| density | 1,65 g/cm3 |
| refractive index | 21 ° (C=10, H2O) |
| solubility | H2O: 50 mg/mL at 20 °C, clear, nearly colorless |
| pka | 4.04, 11.7(at 25℃) |
| PH | 1.0 - 2.5 (25℃, 176g/L in water) |
| PH Range | 1 - 2.5 |
| Odor | Odorless |
| optical activity | [α]25/D 19.0 to 23.0°, c = 10% in H2O |
| Water Solubility | 333 g/L (20 ºC) |
| Stability | Stable. May be weakly light or air sensitive. Incompatible with oxidizing agents, alkalies, iron, copper. |
The starting point for synthesis of vitamin C is the selective of oxidation of the sugar compound D-sorbit to L-sorbose using Acetobacter suboxidans bacteria. L-sorbose is then converted to L-ascorbic acid, better known as vitamin C.
Sodium, potassium, and calcium salts of ascorbic acids are called ascorbates and are used as food preservatives. To make ascorbic acid fat-soluble, it can be esterified. Esters of ascorbic acid and acids, such as palmitic acid to form ascorbyl palmitate and stearic acid to form ascorbic stearate, are used as antioxidants in food, pharmaceuticals, and cosmetics. Ascorbic acid is also essential in the metabolism of some amino acids. It helps protect cells from free radical damage, helps iron absorption, and is essential for many metabolic processes.
Vitamin C is a well-known anti-oxidant. Its effect on free-radical formation when topically applied to the skin by means of a cream has not been clearly established. The effectiveness of topical applications has been questioned due to vitamin C’s instability (it reacts with water and degrades). Some forms are said to have better stability in water systems. Synthetic analogues such as magnesium ascorbyl phosphate are among those considered more effective, as they tend to be more stable. When evaluating its ability to fight free-radical damage in light of its synergistic effect with vitamin e, vitamin C shines. As vitamin e reacts with a free radical, it, in turn, is damaged by the free radical it is fighting. Vitamin C comes in to repair the free-radical damage in vitamin e, allowing e to continue with its free-radical scavenging duties. Past research has indicated that high concentrations of topically applied vitamin C are photoprotective, and apparently the vitamin preparation used in these studies resisted soap and water, washing, or rubbing for three days. More current research has indicated that vitamin C does add protection against uVB damage when combined with uVB sunscreen chemicals. This would lead one to conclude that in combination with conventional sunscreen agents, vitamin C may allow for longer-lasting, broader sun protection. Again, the synergy between vitamins C and e can yield even better results, as apparently a combination of both provides very good protection from uVB damage. However, vitamin C appears to be significantly better than e at protecting against uVA damage. A further conclusion is that the combination of vitamins C, e, and sunscreen offers greater protection than the sum of the protection offered by any of the three ingredients acting alone. Vitamin C also acts as a collagen biosynthesis regulator. It is known to control intercellular colloidal substances such as collagen, and when formulated into the proper vehicles, can have a skin-lightening effect. Vitamin C is said to be able to help the body fortify against infectious conditions by strengthening the immune system. There is some evidence (although debated) that vitamin C can pass through the layers of the skin and promote healing in tissue damaged by burns or injury. It is found, therefore, in burn ointments and creams used for abrasions. Vitamin C is also popular in anti-aging products. Current studies indicate possible anti-inflammatory properties as well.
Physiological antioxidant. Coenzyme for a number of hydroxylation reactions; required for collagen synthesis. Widely distributed in plants and animals. Inadequate intake results in deficiency syndrome s such as scurvy. Used as antimicrobial and antioxidant in foodstuffs.