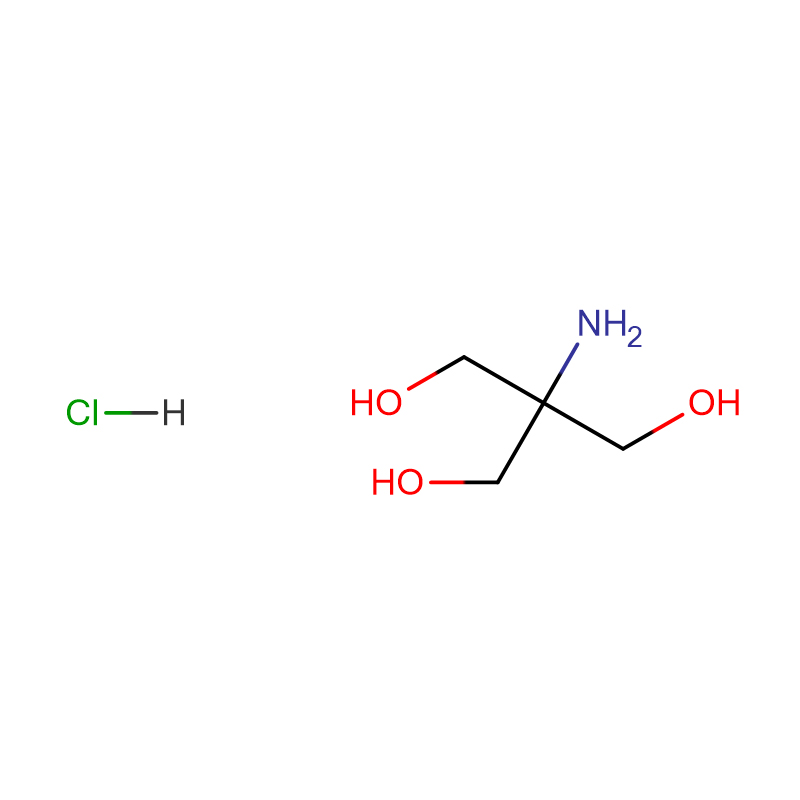
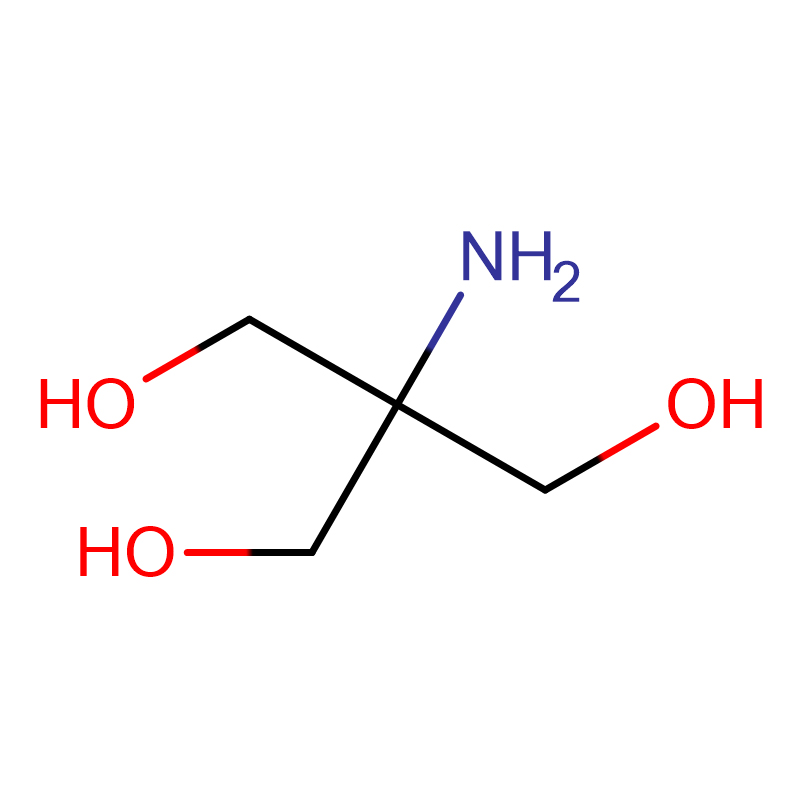
Tris-HCl Cas:1185-53-1 99% White crystalline powder
| Catalog Number | XD90058 |
| Product Name | Tris-HCl |
| CAS | 1185-53-1 |
| Molecular Formula | NH2C(CH2OH)3 · HCl |
| Molecular Weight | 157.60 |
| Storage Details | Ambient |
| Harmonized Tariff Code | 2922190090 |
Product Specification
| Melting Point | 150 - 155 Deg C |
| Water | <0.5% |
| pH | 4.2 - 5 |
| Fe | <5ppm |
| Appearance | White crystalline powder |
| Assay | 99% |
| Trace Metal Analysis | <5ppm |
| For research use only, not for human use | research use only, not for human use |
Introduction: Tris-Hydroxymethylaminomethane hydrochloride is referred to as Tris-HCl. Tris-HCl buffer (Tris-HCl buffer) is not only widely used as a solvent for nucleic acids and proteins, but also has many important uses.
Application: Tris-HCl was used for protein crystal growth at different pH conditions. The low ionic strength of Tris-HCL buffer can be used for the formation of intermediate fibers of lamin in C. elegans. Tris-HCl is also one of the main components of protein electrophoresis buffers. In addition, Tris-HCL is also an intermediate for the preparation of surfactants, vulcanization accelerators and some Chemicalbook drugs. Tris-HCl was also used as a titration standard.
Buffer solution preparation: take tris(hydroxymethyl)aminomethane hydrochloride as the solute, take deionized water as the solvent, and use the HCl aqueous solution with a concentration of 0.1mol/L to adjust the pH value to prepare a trimethylolamine solution with a concentration of 6.057g/L and pH=8.8. Hydroxymethylaminomethane hydrochloride buffer solution (Tris-HCl buffer).
Chemical Properties: White crystals.
Usage: The pH of all buffers is temperature and concentration dependent. For Tris buffer, pH increases by approximately 0.03 units per 1 °C increase in temperature and decreases by 0.03-0.05 units per 10-fold dilution.
Application: for biological buffer
Uses: used as intermediates in organic synthesis