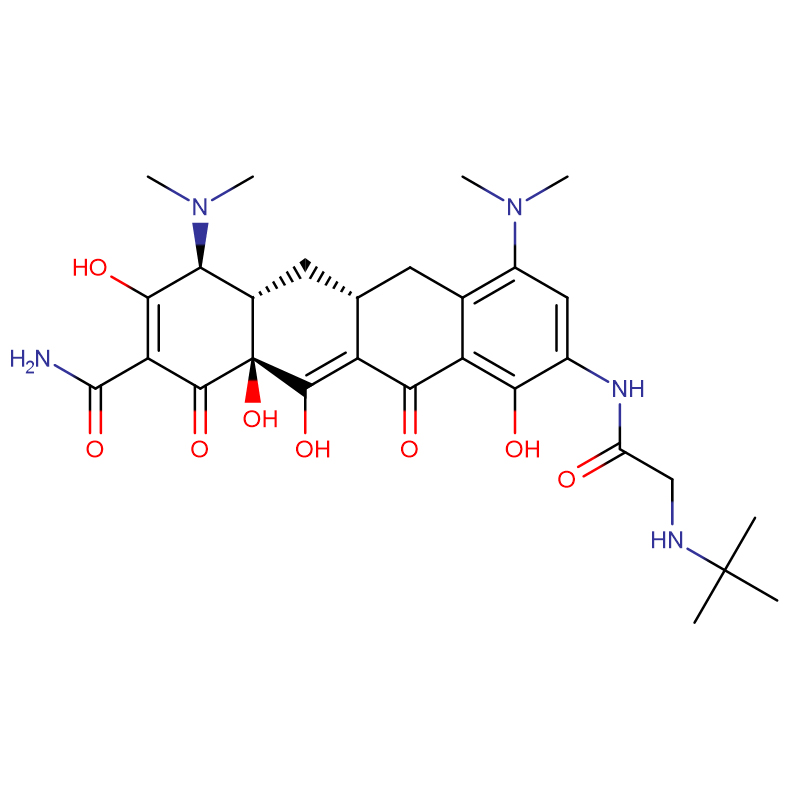
Tigecycline Cas: 220620-09-7
| Catalog Number | XD92381 |
| Product Name | Tigecycline |
| CAS | 220620-09-7 |
| Molecular Formula | C29H39N5O8 |
| Molecular Weight | 585.65 |
| Storage Details | -15 to -20 °C |
| Harmonized Tariff Code | 29419000 |
Product Specification
| Appearance | Orange crystalline powder, odorless, hydroscopic |
| Assay | 99% min |
| Specific rotation | -190°/-230 ° |
| Heavy metals | ≤ 20 ppm |
| pH | 7.0 - 8.5 |
| Residue on Ignition | ≤ 0.1 % |
| Moisture | ≤ 3.0 % |
| Total Impurites | ≤ 2.0 % |
| Clarity and Impurity Absorbance | Clarification and absorbance at 480nm wavelength is less than 0.1 |
| Tigecycline epimers | ≤ 1.0 % |
| Any individual unspecified impurity | ≤ 1.0 % |
Tigecycline is also called 9-tert-glycylaminomycetine or diclofenac, and it is a new type of venous injection antibiotic with broad-spectrum activities. It is a type of 9-tert-glycylaminomycetine derivative and is the first glycylcine antibiotic.
Tigecycline can serve as a second option after failed first-line treatment for multi-drug resistant bacteria, and it is also a new treatment option for patients who are allergic to penicillin or intolerable to other drugs. It can treat patients 18 years old or above with complex skin and skin structure infections or complex abdominal infections such as complex appendicitis, burn infections, abdominal abscesses, deep soft tissue infections, and ulcer infections.
Close