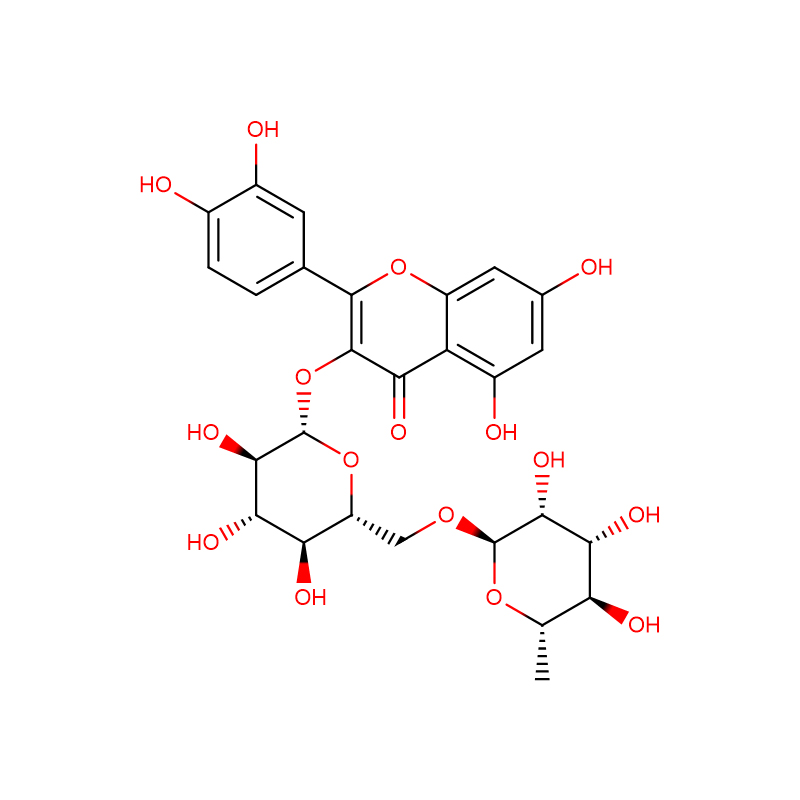
Rutin Cas:153-18-4
| Catalog Number | XD91217 |
| Product Name | Rutin |
| CAS | 153-18-4 |
| Molecular Formula | C27H30O16 |
| Molecular Weight | 610.51 |
| Storage Details | Ambient |
| Harmonized Tariff Code | 2932999099 |
Product Specification
| Appearance | Yellow powder |
| Assay | 99% min |
| Density | 1.3881 (rough estimate) |
| Melting point | 195 ºC |
| Boiling point | 983.1°C at 760 mmHg |
| Refractive index | 1.7650 (estimate) |
| Solubility pyridine: | 50 mg/mL |
| Water soluble | 12.5 g/100 mL |
| Solubility | Soluble in pyridine, formyl and lye, slightly soluble in ethanol, acetone and ethyl acetate, almost insoluble in water, chloroform, ether, benzene, carbon disulfide and petroleum ether. |
Rutin is also called rutoside, quercetin-3-O-rutinoside and sophorin. Rutin powder is extracted from the flower buds of sophora japonica tree. Rutin can regulate blood circulation, reduce blood pressure and blood fat, and it also has anti-inflammatory and anti-allergic effects. Besides, rutin can be used as antioxidant, fortifying agent or natural pigment in food.
Application
1.Rutin inhibits platelet aggregation, as well as decreases capillary permeability, making the blood thinner andimproving circulation. Rutin shows anti-inflammatory activity in some animal.
2.Rutin inhibits aldose reductase activity. Aldose reductase is an enzyme normally present in the eye andelsewhere in the body. It helps change glucose into the sugar alcohol sorbitol.
3.Recent studies show rutin could help prevent blood clots, so could be used to treat patients at risk of heartattacks and strokes.
Function
1.Rutin may modulate the respiratory burst of neutrophils;
2.Rutin is a phenolic antioxidant and has been demonstrated to scavenge superoxide radicals;3.Rutin can promotes circulation, stimulate bile production, help to lower blood cholesterol,and prevent cataracts;
4.Rutin is a bioflavonoid. It can enhance the absorption of Vitamin C; help to relieve pain, bumps and bruises and has an antibacterial effect;
5.Rutin can chelate metal ions, such as ferrous cations. Ferrous cations are involved in the so-called Fenton reaction, which generates reactive oxygen species