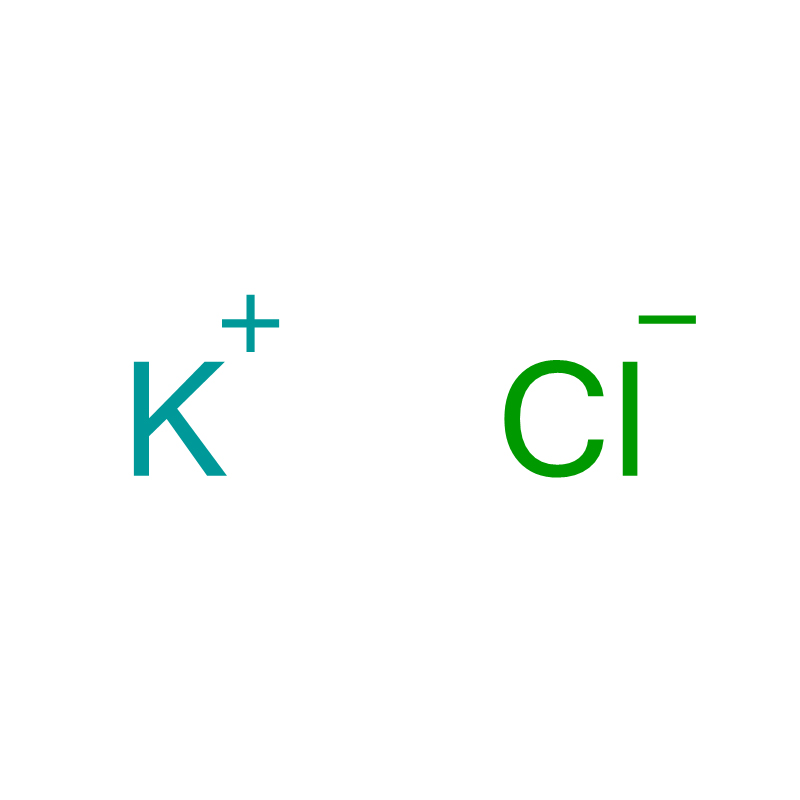Potassium Chloride Cas: 7447-40-7
| Catalog Number | XD91858 |
| Product Name | Potassium Chloride |
| CAS | 7447-40-7 |
| Molecular Formula | ClK |
| Molecular Weight | 74.55 |
| Storage Details | 2-8°C |
| Harmonized Tariff Code | 31042090 |
Product Specification
| Appearance | White crystal powder |
| Assay | 99% min |
| Melting point | 770 °C (lit.) |
| Boiling point | 1420°C |
| density | 1.98 g/mL at 25 °C (lit.) |
| refractive index | n20/D 1.334 |
| Fp | 1500°C |
| solubility | H2O: soluble |
| Specific Gravity | 1.984 |
| Odor | Odorless |
| PH | 5.5-8.0 (20℃, 50mg/mL in H2O) |
| PH Range | 7 |
| Water Solubility | 340 g/L (20 ºC) |
| λmax | λ: 260 nm Amax: 0.02 λ: 280 nm Amax: 0.01 |
| Sensitive | Hygroscopic |
| Sublimation | 1500 ºC |
| Stability | Stable. Incompatible with strong oxidizing agents, strong acids. Protect from moisture. Hygroscopic. |
Potassium chloride (KCl) is used in drug preparations and as a food additive and chemical reagent. It is possible to reduce the sodium in your diet by substituting potassium chloride for table salt (sodium chloride), which may be healthier. Molten potassium chloride is also used in the electrolytic production of metallic potassium. KCl is also found in seawater brine and can be extracted from the mineral carnallite.
Potassium Chloride is a nutrient, dietary supplement, and gelling agent that exists as crystals or powder. it has a solubility of 1 g in 2.8 ml of water at 25°c and 1 g in 1.8 ml of boiling water. hydrochloric acid, and sodium chloride and magnesium chloride diminish its solubility in water. it is used as a salt substitute and mineral supple- ment. it has optional use in artificially sweetened jelly and preserves. it is used as a potassium source for certain types of carrageenan gels. it is used to replace sodium chloride in low-sodium foods.
Potassium chloride is a laboratory reagent used to increase product viscosity in cosmetic and pharmaceutical preparations.
Potassium chloride (KCl), commonly referred to as muriate of potash, is the most common source of potash (K2O), and accounts for about 95 % of world potash production. Virtually all (90 %) commercial potash is extracted from natural sources of potassium salt deposits occurring in thin beds in large salt basins formed by the evaporation of ancient seas. Present-day salt lakes and natural brines represent about 10 % of total recoverable potash. Extraction is followed by milling, washing, screening, flotation, crystallization, refining and drying.
More than 90 % of the total KCl consumption is used for fertilizer production. Production of potassium hydroxide accounts for more than 90 % of the non-fertilizer or industrial use of KCl. KOH is also used in the production of some agricultural-grade liquid fertilizers. uses of KCl include:
Potassium chloride (KCl) is inorganic salt used for making fertilizers, since the growth of many plants is limited by their potassium intake. Potassium in plants is important for the osmotic and ionic regulation, plays a key role in the water homeostasis and is closely connected with processes involved in the protein synthesis.
In photography. In buffer solutions, electrode cells.
Potassium chloride may be used for the preparation of phosphate buffered saline, and for the extraction and solubilization of proteins.
Used in buffer solutions, medicine, scientific applications, and food processing.
Used in nutritent; gelling agent; salt substitute; yeast food.
food/foodstuff additives: KCl is used as a nutrient and/or dietary supplement food additive. KCl also serves as a potassium supplement of animal feed.
pharmaceutical products: KCl is an important therapeutic agent, which is used mainly in the treatment of hypokalemia and associated conditions. Hypokalemia (potassium deficiency) is a potentially fatal condition in which the body fails to retain sufficient potassium to maintain health.
laboratory chemicals: KCl is used in electrode cells, buffer solutions, and spectroscopy.
drilling mud for oil production industry: KCl is used as conditioner in oil drilling muds and as a shale stabilizer to prevent swelling.
flame retardants and fire preventing agents: KCl is used as a component in dry chemical fire extinguisher.
anti-freezing agents: KCl is used to melt ice on streets and driveways.
About 4-5% of potash production is used in industrial applications (UNIDOIFDC, 1998). In 1996, the world supply of industrial grade potash was close to 1.35 Mt K2O. This industrial material is 98-99% pure, compared with the agricultural potash specification of 60% K2O minimum (equivalent to 95% KCl). Industrial potash should contain at least 62% K2O and have very low levels of Na, Mg, Ca, SO4 and Br. This high-grade potash is produced by only a few producers in worldwide.
Potassium hydroxide (KOH), also known as caustic potash, is the largestvolume K product for non-fertilizer use. It is produced by the electrolysis of industrial KCl and is widely used for manufacturing soaps, detergents, grease, catalysts, synthetic rubber, matches, dyes and insecticides. Caustic potash is also as a liquid fertilizer and as an ingredient in alkaline batteries and photographic film processing chemicals.
Potassium hydroxide is a raw material in the production of various K salts, mainly K carbonates, and also citrates, silicates, acetates, etc. Potassium carbonate confers excellent clarity to glass thus is used for most fine optical lenses, eyeglasses, fine crystal, glassware, chinaware and TV tubes. Potassium bicarbonate is used largely in the food and pharmaceutical industries.
Potash-derived compounds and salts are also used in the production of metal fluxes, cured meats, tempered steel, paper fumigants, case hardened steel, bleaching agents, baking powder, cream of tartar and beverages. Worldwide, industrial KCl is estimated to be used as follows: detergents and soaps, 30-35%; glass and ceramics, 25-28%; textiles and dyes 20-22%; chemicals and drugs, 13-15%; and other uses, 7-5% (UNIDO-IFDC, 1998).
Potassium chloride is a widely used reagent in biochemistry and molecular biology. It is a component of phosphate buffered saline (PBS, Product No. P 3813) and of polymerase chain reaction (PCR) buffer (50 mM KCl).
KCl is also used in studies of ion transport and potassium channels.
KCl is also utilized in the solubilization, extraction, purification, and crystallization of proteins.
The use of KCl in the crystallization of histone core octamers has been reported.