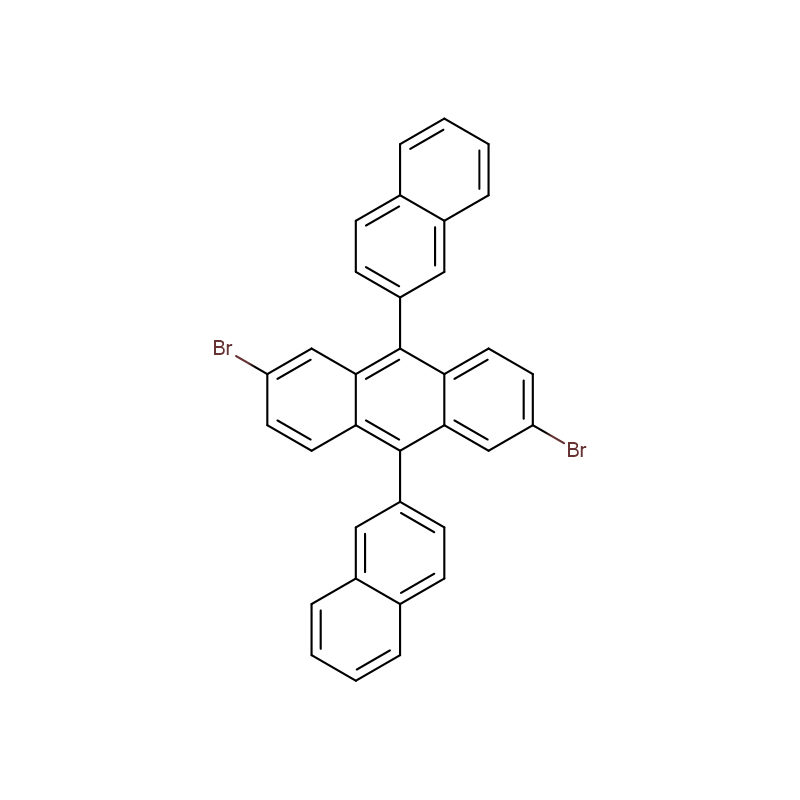
Polystyrene sulfonic acid CAS:28210-41-5
| Catalog Number | XD90753 |
| Product Name | Polystyrene sulfonic acid |
| CAS | 28210-41-5 |
| Molecular Formula | C8H8O3S |
| Molecular Weight | 184.212 |
| Storage Details | Ambient |
Product Specification
| Appearance | Pure liquid |
| Assay | 99% |
Hyperkalemia during renin-angiotensin-aldosterone system inhibition (RAAS-I) may prevent optimum dosing. Treatment options include sodium polystyrene sulfonate potassium binding resins, but safety and efficacy concerns exist, including associated colonic necrosis (CN). Alternative agents have been studied, but cost-utility has not been estimated.We performed a cost-utility analysis of outpatients ≥ 18 years of age receiving chronic RAAS-I, with a history of hyperkalemia or chronic kidney disease, prescribed either sodium polystyrene sulfonate or a theoretical "drug X" binding resin for chronic hyperkalemia. Data were obtained from existing literature. We used a decision analytic model with Monte Carlo probabilistic sensitivity analyses, from a health care payer perspective and a 12-month time horizon. Costs were measured in US dollars. Effectiveness was measured in quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs).Drug X could cost no more than $ 10.77 per daily dose to be cost-effective, at a willingness-to- pay (WTP) threshold of $ 50,000/QALY. At $ 40.00 per daily dose, drug X achieved an incremental cost effectiveness ratio of $26,088,369.00 per QALY gained. One-way sensitivity analysis showed sodium polystyrene sulfonate to be the cost-effective option for CN incidences ≤ 19.9%. Limitations include incomplete information on outpatient outcomes and lack of data directly comparing sodium polystyrene sulfonate to potential alternatives.Alternatives may not be cost-effective unless priced similarly to sodium polystyrene sulfonate. This analysis may guide decisions regarding adoption of alternative agents for chronic hyperkalemia control, and suggests that sodium polystyrene sulfonate be employed as an active control in clinical trials of these agents.


![[4-(4-Aminobenzoyl)oxyphenyl] 4-aminobenzoate CAS:22095-98-3](https://cdn.globalso.com/xdbiochems/22095-98-3.jpg)
![Thieno[3,2-b]thiophene Cas:251-41-2 White to Gray to Brown powder to crystal](https://cdn.globalso.com/xdbiochems/251-41-2.jpg)