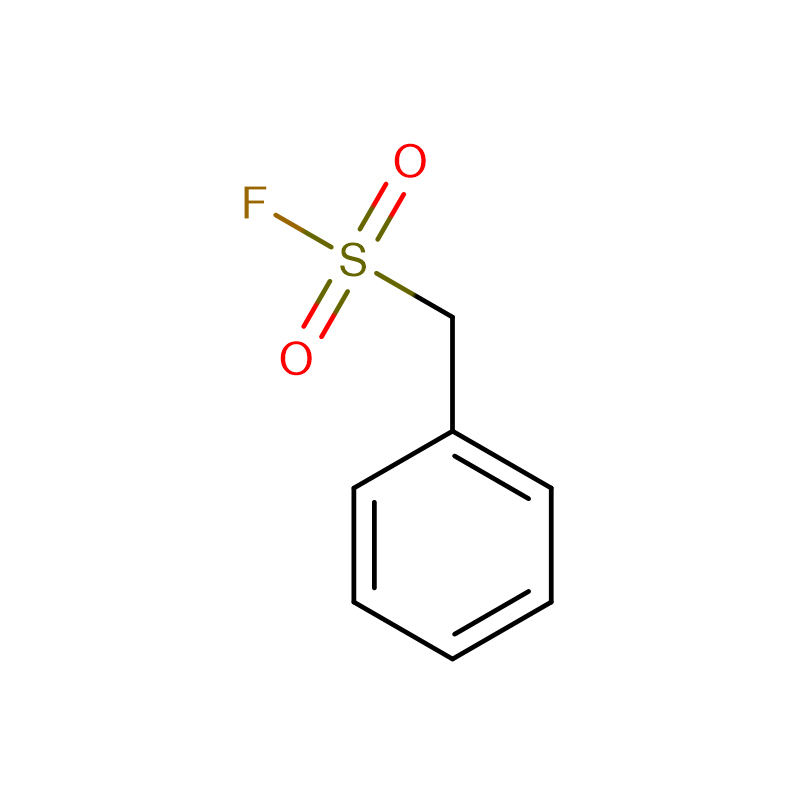
PMSF Cas: 329-98-6 98.0% white crystalline powder Phenylmethanesulfonyl fluoride (PMSF)
| Catalog Number | XD90250 |
|
Product Name |
Phenylmethanesulfonyl fluoride (PMSF) |
|
CAS |
329-98-6 |
|
Molecular Formula |
C7H7FO2S |
|
Molecular Weight |
174.1927 |
| Storage Details | Ambient |
|
Harmonized Tariff Code |
29049900 |
Product Specification
| Assay | ≥98.0% HPLC |
| Appearance | white crystalline powder |
PMSF is an irreversible serine/cysteine protease inhibitor commonly used in the preparation of cell lysates.
In vitro studies: PMSF (2 mM) inhibited carbachol-stimulated inositol phosphate accumulation by only 15%-19% in the presence of Li+. The inhibition of phosphoinositide turnover by PMSF is due to one or more steps following the breakdown of phosphoinositides [1]. PMSF inhibits acylation of inositol residues of GPI intermediates in the bloodstream of T. brucei. PMSF inhibits glycolipid C formation but not fatty acid remodeling in vitro. PMSF inhibits addition of GPI acylation and ethanolamine phosphatase in procyclic trypanosomes, but not in Hela cells [2].
In vivo studies: PMSF (0.1 mL/10 g b.wt, ip) produced antinociception, as shown by a dose-responsive increase in %MPE in tail-flick latency assessment, but failed to produce clear dose-responsive motor inhibition. Mice that received an intraperitoneal injection of PMSF exhibited cannabinoid effects including antinociception, hypothermia and immobility with ED50 values of 86, 224 and 206 mg/kg, respectively. PMSF (30 mg/kg) pretreatment enhances the effects of anandamide on tail-flick responses (antinociception), locomotor activity and mobility by 5-fold, 10-fold and 8-fold, respectively[3].
Animal experiments: Male ICR mice weighing 18 to 25 g were used in the assay. PMSF was dissolved in sesame oil and administered intraperitoneally in a volume of 0.1 mL/10 g b.wt. Always administer PMSF 10 minutes prior to intravenous anandamide or vehicle injection. Mice were acclimated to the evaluation room overnight without interruption of food or water. Following intravenous anandamide or vehicle administration, each animal was evaluated as follows: 5 minutes for tail-flick latency (antinociceptive) responses and 5 to 15 minutes for spontaneous (motor) activity; or 5 minutes for core (rectal) temperature and ring Immobilization (catalepsy) for 5 to 10 minutes.