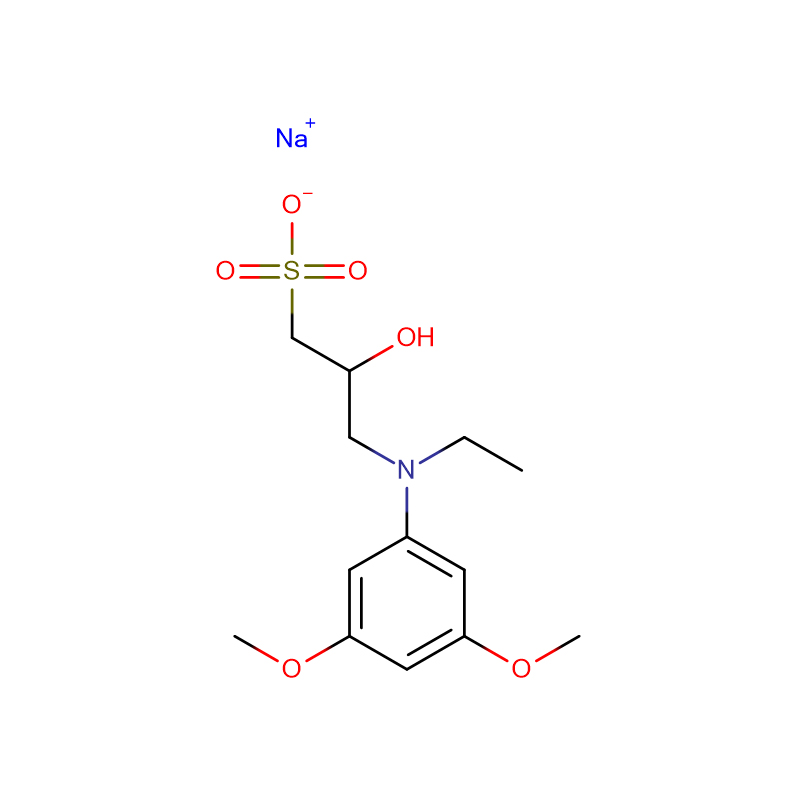
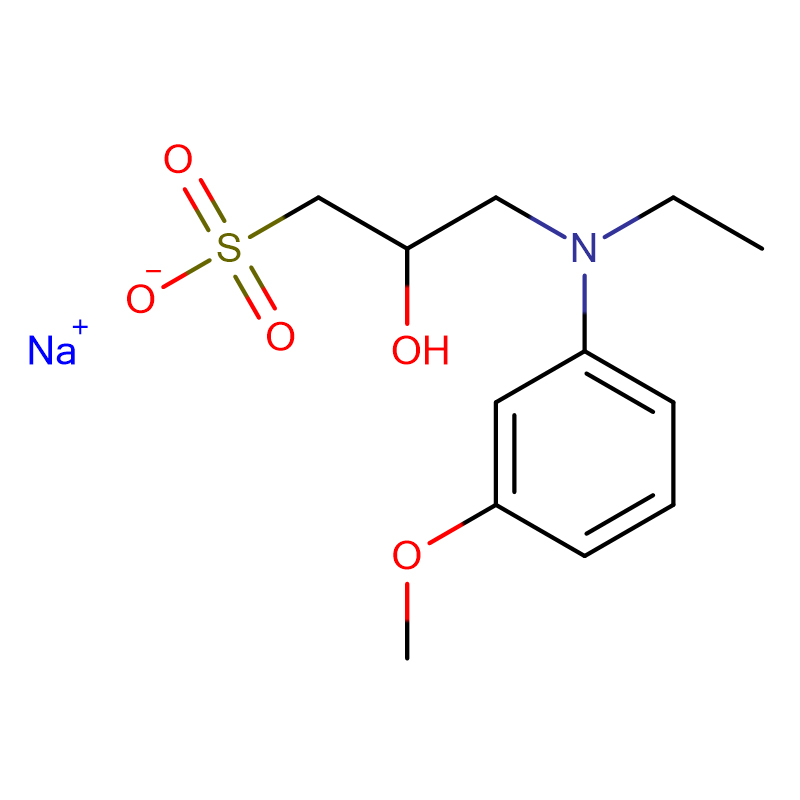
PIPES sesquisodium salt Cas:100037-69-2 Sodium 1,4- piperazinediethanesulfonate White powder 99%
| Catalog Number | XD90094 |
| Product Name | PIPES sesquisodium salt |
| CAS | 100037-69-2 |
| Molecular Formula | C8H16.5N2Na1.5O6S2 |
| Molecular Weight | 335.34 |
| Storage Details | Ambient |
| Harmonized Tariff Code | 29335995 |
Product Specification
| Appearance | White powder |
| Assay | 99% min |
| Storage Temp | Store at RT |
| pH | 6.5 - 7.1 |
| Water Content | 3% max |
At the most basic level, organic compounds are compounds that contain carbon and hydrogen. These compounds are called organic compounds because they were once thought to be derived from living things, but this is not the case. There are millions of organic compounds that occur naturally or can be produced synthetically. Examples of organic compounds are carbohydrates, fats (lipids), proteins and nucleic acids, which are the basis of the molecules of life. Organic compounds also include oil and natural gas, which are major constituents of fossil fuels.
Chemicals are all chemical compounds produced by chemical processes in the lab or industrially. They can be pure substances or mixtures of substances. Chemicals are divided into organic and inorganic chemicals. Organic chemistry covers practically all the carbon-containing compounds, while inorganic chemistry (inorganic matter) relates to the other elements of the periodic table and their compounds. Petrochemicals are an important subsection of organic chemistry.
It wasn’t until the mid-19th century that scientists realized that carbon content is the defining characteristic of an organic compound, and the boundaries of the two disciplines – organic and inorganic chemistry – are now becoming increasingly blurred. Nevertheless, a distinction is still useful because the reaction mechanisms and material structures often differ in inorganic and organic chemistry.
Organic chemistry covers approximately 19 million known carbon compounds, greatly exceeding the number of known inorganic compounds (about 500,000). This is attributable to carbon’s special ability to form branched chains and ring structures with other carbon atoms. The naming of organic compounds is determined by International Union of Pure and Applied Chemistry (IUPAC) rules and are is set out in its “Blue Book.”