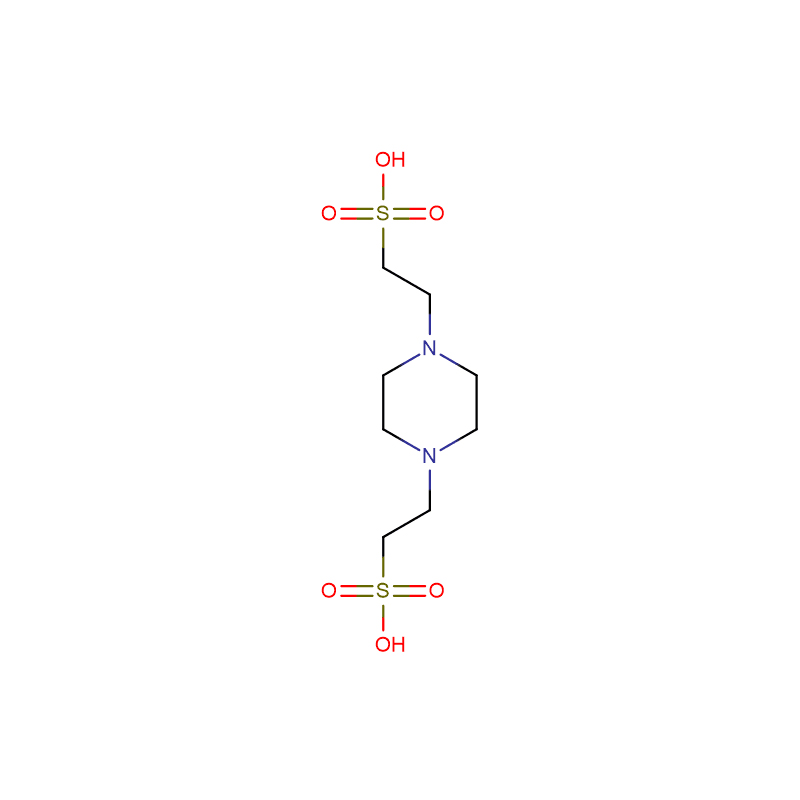
PIPES Cas: 5625-37-6 White crystalline powder 99% ABTS DIAMMONIUM SALT ULTRA PURE GRADE
| Catalog Number | XD90117 |
|
Product Name |
PIPES (Piperazine-1,4-bis(2-ethanesulfonic acid)) |
|
CAS |
5625-37-6 |
|
Molecular Formula |
C8H18N2O6S2 |
|
Molecular Weight |
302.37 |
|
Storage Details |
Ambient |
|
Harmonized Tariff Code |
2933599 |
Product Specification
|
Heavy metals |
<5ppm |
|
Loss on Drying |
<1.0% |
|
Solubility |
Clear, Colourless solution (5% 1N NaOH) |
|
Assay |
99 - 101% |
|
Appearance |
White crystalline powder |
PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] is frequently used as a buffering agent in biochemistry. It is an ethanesulfonic acid buffer developed by Good et al. in the 1960s. PIPES has a pKa near the physiological pH which makes it useful in cell culture work. It has been documented to minimize lipid loss when buffering glutaraldehyde histology in plant and animal tissues.A buffering agent with a pKa near physiological pH.
Antimicrobial peptide magainin 2 forms pores in lipid membranes and induces membrane permeation of the cellular contents. Although this permeation is likely the main cause of its bactericidal activity, the mechanism of pore formation remains poorly understood. We therefore investigated in detail the interaction of magainin 2 with lipid membranes using single giant unilamellar vesicles (GUVs). The binding of magainin 2 to the lipid membrane of GUVs increased the fractional change in the area of the membrane, δ, which was proportional to the surface concentration of magainin 2, X. This indicates that the rate constant of the magainin 2-induced two-state transition from the intact state to the pore state greatly increased with an increase in δ. The tension of a lipid membrane following aspiration of a GUV also activated magainin 2-induced pore formation. To reveal the location of magainin 2, the interaction of carboxyfluorescein (CF)-labeled magainin 2 (CF-magainin 2) with single GUVs containing a water-soluble fluorescent probe, AF647, was investigated using confocal microscopy. In the absence of tension due to aspiration, after the interaction of magainin 2 the fluorescence intensity of the GUV rim due to CF-magainin 2 increased rapidly to a steady value, which remained constant for a long time, and at 4-32 s before the start of leakage of AF647 the rim intensity began to increase rapidly to another steady value. In contrast, in the presence of the tension, no increase in rim intensity just before the start of leakage was observed. These results indicate that magainin 2 cannot translocate from the outer to the inner monolayer until just before pore formation. Based on these results, we conclude that a magainin 2-induced pore is a stretch-activated pore and the stretch of the inner monolayer is a main driving force of the pore formation.