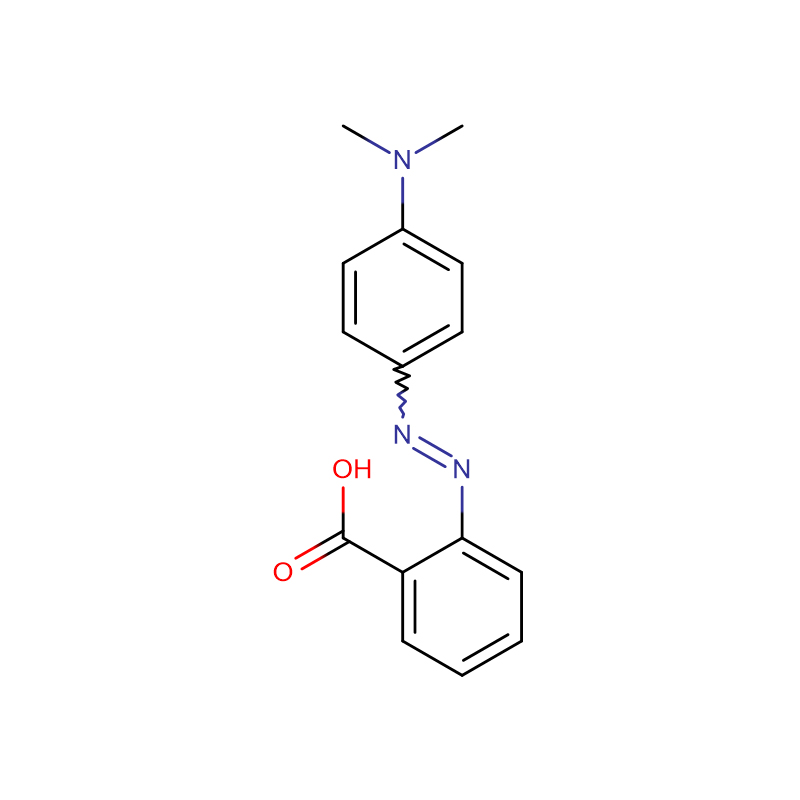
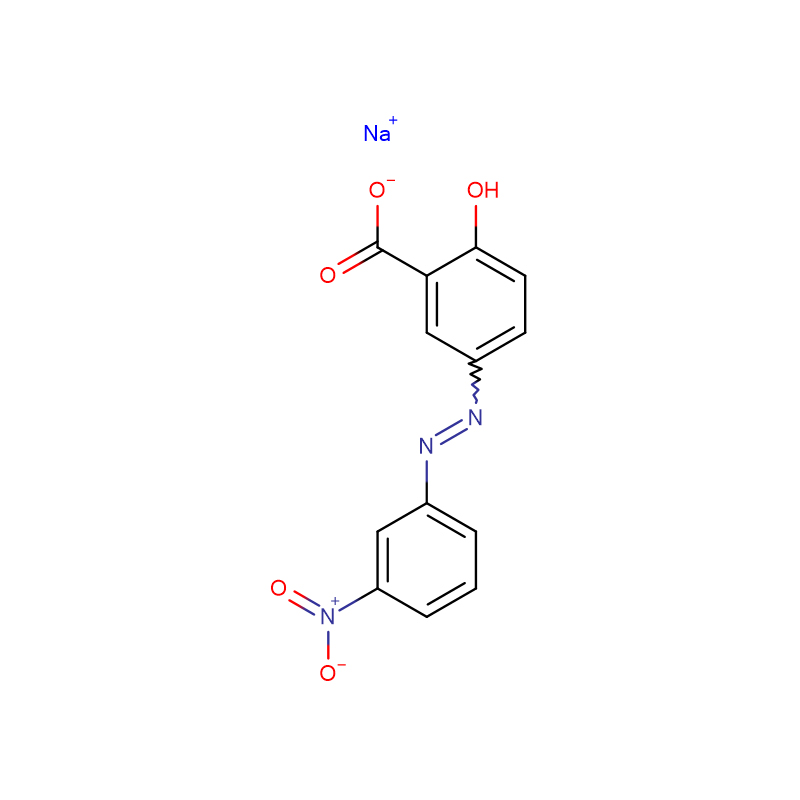
Methyl Red CAS:493-52-7
| Catalog Number | XD90492 |
| Product Name | Methyl Red |
| CAS | 493-52-7 |
| Molecular Formula | C15H15N3O2 |
| Molecular Weight | 269.30 |
| Storage Details | Ambient |
| Harmonized Tariff Code | 29270000 |
Product Specification
| Appearance | Violet crystals |
| Assay | 99% |
| Loss on Drying | 3% max |
| Transition Range | PH 4.2 - 6.2 Pink-Yellow |
| Solubility at 0.1% (95% Ethanol) | Clear Red Solution |
| Absorption Maximum (pH 4.2) λ1 max | 523-528 nm |
| Absorption Maximum (pH 6.2) λ2 max | 430-435 nm |
| Absorbtivity (A1%/1cm at pH 4.2, at λ1 max ) | 1300-1400 |
| Absorbtivity (A1%/1cm at pH 6.2, at λ2 max ) | 700-800 |
Chemical Properties: Glossy purple crystal or reddish-brown powder. Melting point 180-182 ℃. Soluble in ethanol, glacial acetic acid, almost insoluble in water.
Uses: Methyl red is one of the commonly used acid-base indicators, the usual concentration is 0.1% ethanol solution, pH4.4 (red)-6.2 (yellow). Also used for in vivo staining of protozoa.
Purpose: Protozoa living dyeing; acid-base indicator, pH discoloration range of 4.4 (red) to 6.2 (yellow); titration of ammonia, weak organic bases and alkaloids, but not suitable for organic acids other than oxalic acid and picric acid; can be combined with Bromocresol green and methylene blue form a mixed indicator to shorten the color change range and improve the sensitivity of color change; adsorption indicator for precipitation titration, such as titration of fluoride ion with thorium nitrate; determination of free chlorine, chlorite and other oxidants
Uses: protozoa live staining, acid-base indicator (pH4.4to6.2), clinical serum protein biochemical test. The pH discoloration range is 4.4 (red)-6.2 (yellow). Titrate ammonia, weak organic bases and alkaloids, but not for organic acids other than oxalic and picric acids. It can be combined with bromocresol green and methylene blue to form a mixed indicator to shorten the color change range and improve the sensitivity of color change. Adsorption indicators for precipitation titrations, such as fluoride titration with thorium nitrate. Determination of free chlorine, chlorite and other oxidants.