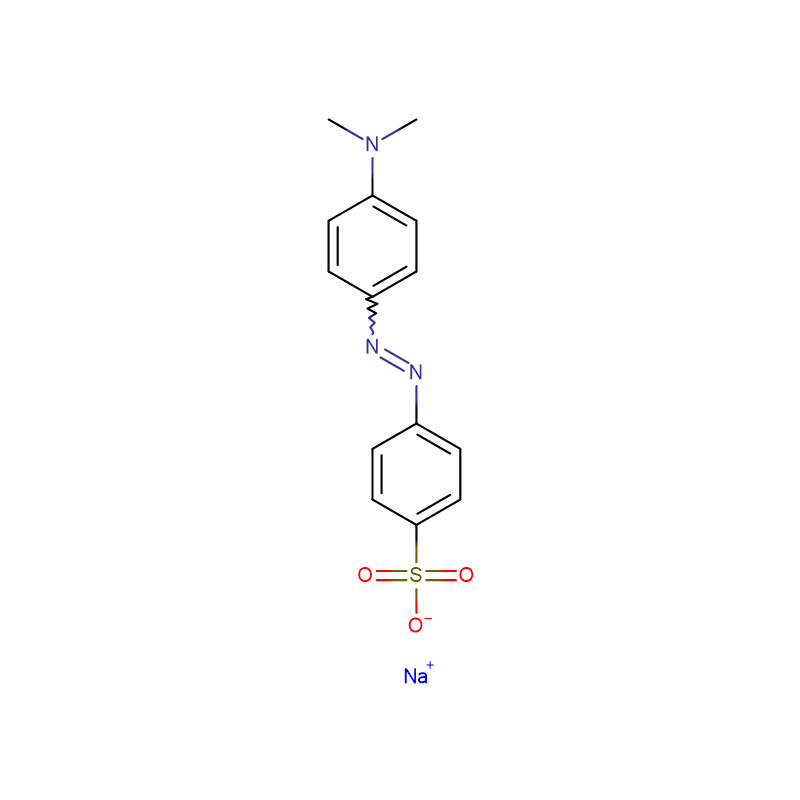
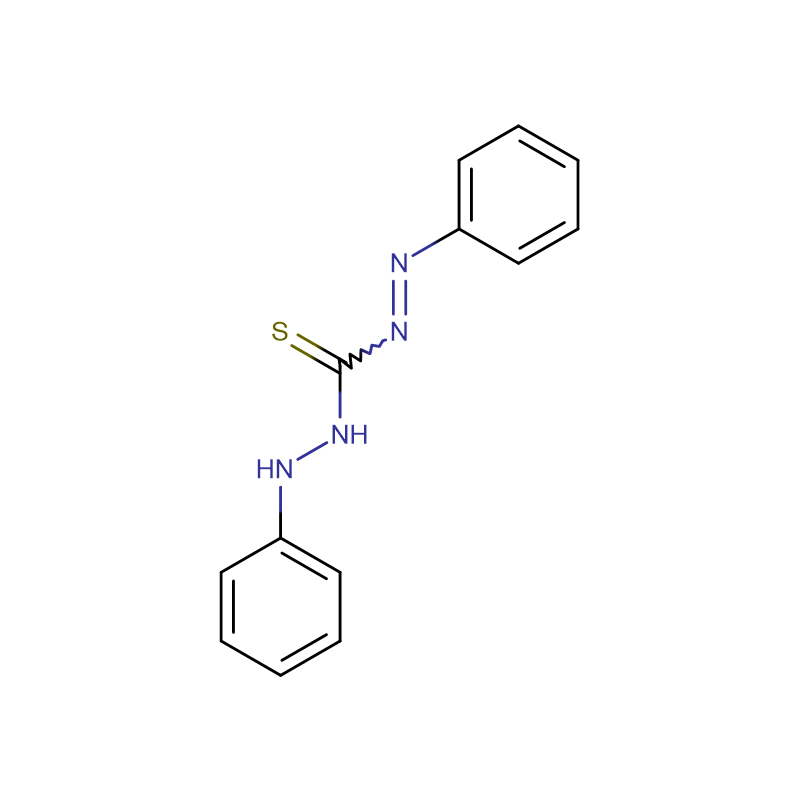
Methyl Orange CAS:547-58-0
| Catalog Number | XD90490 |
| Product Name | Methyl Orange |
| CAS | 547-58-0 |
| Molecular Formula | C14H14N3NaO3S |
| Molecular Weight | 327.33 |
| Storage Details | Ambient |
| Harmonized Tariff Code | 29270000 |
Product Specification
| Appearance | Orange/ yellow powder |
| Assay | 99% |
| pH | 3-4.4 |
| Loss on Drying | <5.0% |
| Dye Content | >=95% |
Application: Methyl orange has been used in laboratory and industrial and agricultural production for pH control of chemical reactions and acid-base titration analysis of chemical products and intermediates. In the printing and dyeing industry, the pH remaining on the fabric should be measured with an indicator and washed to make the fabric neutral. If there is acidity on Chemicalbook fruit cloth, it will affect its color and fastness when dyed or printed with reactive dyes. The disadvantage of methyl orange indicator is that the yellow-red color is more difficult to identify, and it has now been replaced by a wide range of indicators. Methyl orange is also an azo dye that can be used to print and dye textiles
Acid-base titration indicator "Methyl orange is a commonly used acid-base titration indicator in analytical chemistry. Its concentration of 0.1% aqueous solution has a pH of 3.1 (red) to 4.4 (yellow). It is suitable for strong acid and strong base, Titration between weak bases. It exists in the form of sulfonic acid sodium salt in neutral or alkaline solution, and is converted into sulfonic acid in acidic solution, so that the acidic sulfonic acid group forms with the basic dimethylamino group in the molecule. The inner salt form (paraquinone structure) of p-dimethylaminophenylazobenzenesulfonic acid becomes a conjugated system containing a p-quinone structure, so the color changes accordingly, and it is not suitable for titration of organic acid compounds It is also used for spectrophotometric determination of chlorine, bromine and bromide ions. It is also used for biological dyeing, etc. Methyl orange has been used in laboratory and industrial and agricultural production for pH control of chemical reactions and acid for chemical products and intermediates Alkali titration analysis. In the printing and dyeing industry, the residual pH on the fabric should be measured with an indicator and washed to make the fabric neutral. If the fabric has acidity, it will affect its color and color when dyed or printed with reactive dyes. Fastness. The disadvantage of methyl orange indicator is that the yellow-red color is more difficult to identify, and it has now been replaced by a wide range of indicators (see "phenolphthalein"). Methyl orange is also an azo dye, which can be used for printing and dyeing textiles.
Uses: As acid-base indicator, pH3.1 (red)-4.4 (yellow), also used for biological dyeing.
Uses: acid-base indicator, pH discoloration range from 3.1 (red) to 4.4 (yellow), determination of the alkalinity of most mineral acids, strong bases and water; volumetric determination of tin (Sn2+ fades methyl orange when heated); strong reduction Decolorizing indicator for oxidants (Ti3+, Cr2+) and strong oxidants (chlorine, bromine); spectrophotometric determination of chlorine, bromine and bromide ions; it can be combined with sodium indigo disulfonate or bromocresol green to form a mixed indicator to shorten discoloration Domain and enhance the sharpness of color change; redox indicators such as trivalent arsenic or antimony for potassium bromate titration
Uses: Acid-base indicator, achromatic indicator of strong reducing agent and strong oxidant, cytoplasmic indicator, histological contrast stain, pollen tube staining. The pH value changes color range from 3.1 (red) to 4.4 (yellow), and determines the alkalinity of most mineral acids, strong alkalis and water. Volumetric determination of tin (Sn2+ discolors methyl orange when hot). Decolorizing indicator for strong reducing agents (Ti3+), Cr2+) and strong oxidizing agents (chlorine, bromine). Spectrophotometric determination of chlorine, bromine and bromide ions. It can be combined with sodium indigo disulfonate or bromocresol green to form a mixed indicator to shorten the color change range and improve the sharpness of color change. Redox indicators such as trivalent arsenic or antimony for potassium bromate titration.