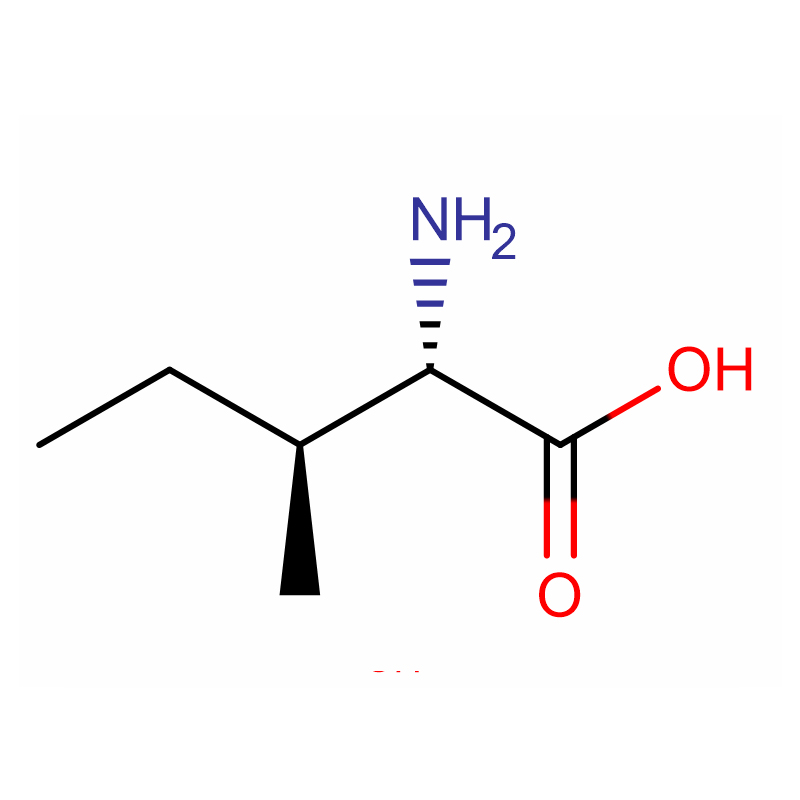
L-Isoleucine Cas: 73-32-5 98.5-101.5% White powder
| Catalog Number | XD90303 |
| Product Name | L-Isoleucine |
|
CAS |
73-32-5 |
|
Molecular Formula |
C6H13NO2 |
|
Molecular Weight |
131.17292 |
| Storage Details | Ambient |
| Harmonized Tariff Code | 29224985 |
Product Specification
| Specific rotation | +38.9 to +41.8 |
| Heavy metals | <15ppm |
| AS | <1.5ppm |
| Ph | 5.5 - 7 |
| Loss on Drying | <0.3% |
| Sulfate | <0.03% |
| Assay | 99% |
| Iron | <30ppm |
| Residue on Ignition | <0.3% |
| Cl | <0.05% |
| Appearance | White/ off white powder |
Genome evolution in intracellular microbial symbionts is characterized by gene loss, generating some of the smallest and most gene-poor genomes known. As a result of gene loss these genomes commonly contain metabolic pathways that are fragmented relative to their free-living relatives. The evolutionary retention of fragmented metabolic pathways in the gene-poor genomes of endosymbionts suggests that they are functional. However, it is not always clear how they maintain functionality. To date, the fragmented metabolic pathways of endosymbionts have been shown to maintain functionality through complementation by host genes, complementation by genes of another endosymbiont and complementation by genes in host genomes that have been horizontally acquired from a microbial source that is not the endosymbiont. Here, we demonstrate a fourth mechanism.We investigate the evolutionary retention of a fragmented pathway for the essential nutrient pantothenate (vitamin B5) in the pea aphid, Acyrthos iphon pisum endosymbiosis with Buchnera aphidicola. Using quantitative analysis of gene expression we present evidence for complementation of the Buchnera pantothenate biosynthesis pathway by host genes. Further, using complementation assays in an Escherichia coli mutant we demonstrate functional replacement of a pantothenate biosynthesis enzyme, 2-dehydropantoate 2-reductase (E.C. 1.1.1.169), by an endosymbiont gene, ilvC, encoding a substrate ambiguous enzyme.Earlier studies have speculated that missing enzyme steps in fragmented endosymbiont metabolic pathways are completed by adaptable endosymbiont enzymes from other pathways. Here, we experimentally demonstrate completion of a fragmented endosymbiont vitamin biosynthesis pathway by recruitment of a substrate ambiguous enzyme from another pathway. In addition, this work extends host/symbiont metabolic collaboration in the aphid/Buchnera symbiosis from amino acid metabolism to include vitamin biosynthesis.