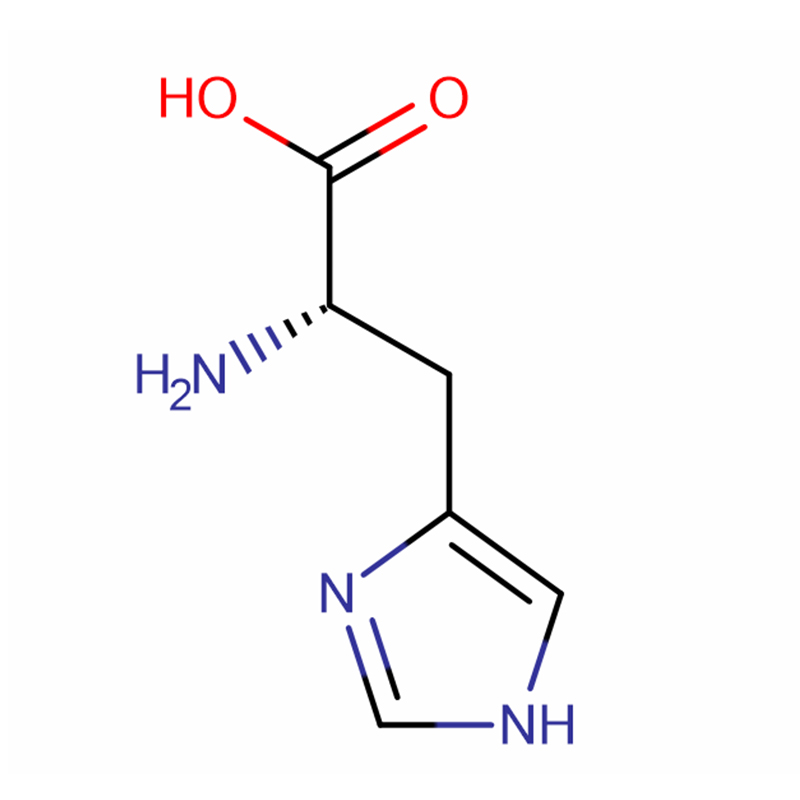
L-Histidine Cas: 71-00-1 98.5% White crystals or crystalline powder
| Catalog Number | XD90301 |
| Product Name | L-Histidine |
|
CAS |
71-00-1 |
|
Molecular Formula |
C6H9N3O2 |
|
Molecular Weight |
155.15 |
| Storage Details | Ambient |
|
Harmonized Tariff Code |
29332990 |
Product Specification
| Assay | 98.5% min |
| Appearance | White crystals or crystalline powder |
| Grade | USP39 |
| Specific rotation | +12.6 ° to +14.0 ° |
| Identification | Infrared absorption |
| pH | 7.0 - 8.5 |
| SO4 | <=0.03% |
| Fe | <=30ppm |
| Loss on Drying | <=0.2% |
| Residue on Ignition | <=0.1% |
| Cl | <=0.05% |
| Heavy metals ( PB ) | <=15ppm |
| Related componds | Not more than 0.5% of any individual impurity is found; Not more than 2.0% of total impurities is found |
he quadruplex forming G-rich sequences are unevenly distributed throughout the human genome. Their enrichment in oncogenic promoters and telomeres has generated interest in targeting G-quadruplex (GQ) for an anticancer therapy. Here, we present a quantitative analysis on the conformations and dynamics of GQ forming sequences measured by single molecule fluorescence. Additionally, we relate these properties to GQ targeting ligands and G4 resolvase 1 (G4R1) protein binding. Our result shows that both the loop (non-G components) length and sequence contribute to the conformation of the GQ. Real time single molecule traces reveal that the folding dynamics also depend on the loop composition. We demonstrate that GQ-stabilizing small molecules, N-methyl mesoporphyrin IX (NMM), its analog, NMP and the G4R1 protein bind selectively to the parallel GQ conformation. Our findings point to the complexity of GQ folding governed by the loop length and sequence and how the GQ conformation determines the small molecule and protein binding propensity.