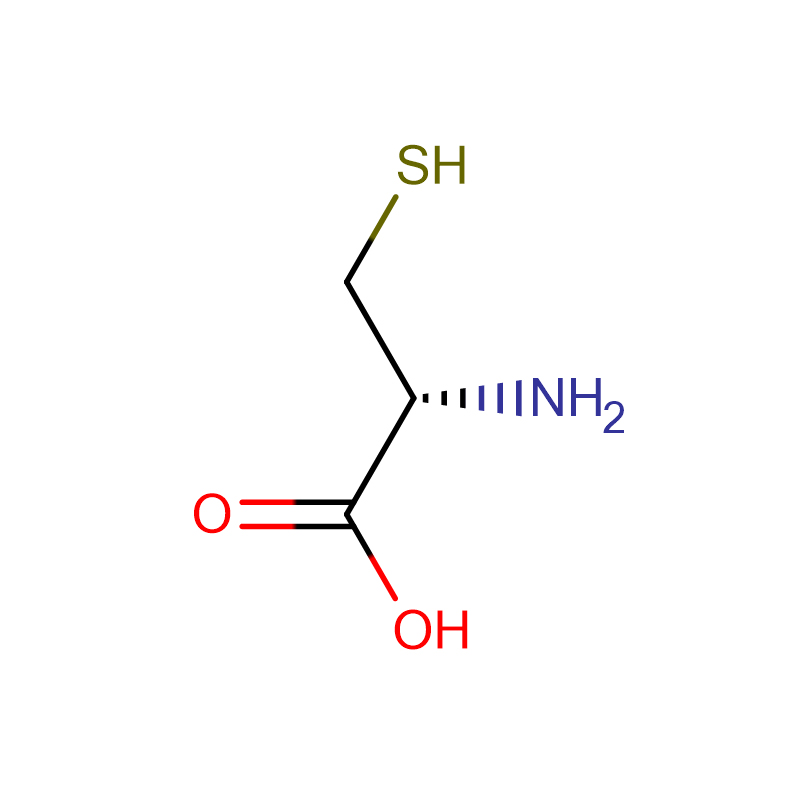
L-Cysteine CAS:52-90-4 98-101% White crystalline powder
| Catalog Number | XD90321 |
| Product Name | L-Cysteine |
| CAS | 52-90-4 |
| Molecular Formula | HSCH2CH(NH2)CO2H |
| Molecular Weight | 121.16 |
| Storage Details | 2 to 8 °C |
| Harmonized Tariff Code | 29309013 |
Product Specification
| Appearance | White crystalline powder |
| Assay | 98.0% - 101.0% |
| Specific rotation | +8.3°~ +9.5° |
| pH | 4.5 ~ 5.5 |
| SO4 | 0.03% max |
| Fe | 10ppm max |
| Loss on Drying | 0.5% max |
| Residue on Ignition | 0.1% max |
| NH4 | 0.02% max |
| AS2O3 | 1ppm max |
| Cl | ≤0.2% |
| Heavy metals (as Pb) | 10ppm max |
| State of Solution | 95.0% min |
Iron-sulfur clusters function as cofactors of a wide range of proteins, with diverse molecular roles in both prokaryotic and eukaryotic cells. Dedicated machineries assemble the clusters and deliver them to the final acceptor molecules in a tightly regulated process. In the prototypical Gram-negative bacterium Escherichia coli, the two existing iron-sulfur cluster assembly systems, iron-sulfur cluster (ISC) and sulfur assimilation (SUF) pathways, are closely interconnected. The ISC pathway regulator, IscR, is a transcription factor of the helix-turn-helix type that can coordinate a [2Fe-2S] cluster. Redox conditions and iron or sulfur availability modulate the ligation status of the labile IscR cluster, which in turn determines a switch in DNA sequence specificity of the regulator: cluster-containing IscR can bind to a family of gene promoters (type-1) whereas the clusterless form recognizes only a second group of sequences (type-2). However, iron-sulfur cluster biogenesis in Gram-posi tive bacteria is not so well characterized, and most organisms of this group display only one of the iron-sulfur cluster assembly systems. A notable exception is the unique Gram-positive dissimilatory metal reducing bacterium Thermincola potens, where genes from both systems could be identified, albeit with a diverging organization from that of Gram-negative bacteria. We demonstrated that one of these genes encodes a functional IscR homolog and is likely involved in the regulation of iron-sulfur cluster biogenesis in T. potens. Structural and biochemical characterization of T. potens and E. coli IscR revealed a strikingly similar architecture and unveiled an unforeseen conservation of the unique mechanism of sequence discrimination characteristic of this distinctive group of transcription regulators.