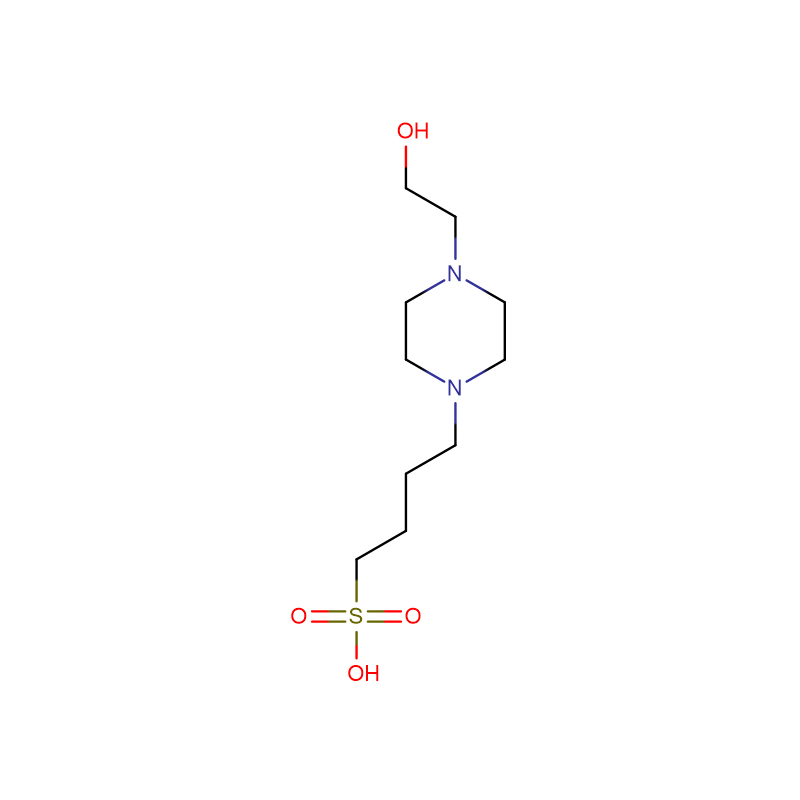
HEPBS Cas:161308-36-7 N- (2- Hydroxyethyl) piperazine- N’- (4- butanesulfonic acid) White crystalline powder 99%
| Catalog Number | XD90100 |
| Product Name | HEPBS |
| CAS | 161308-36-7 |
| Molecular Formula | C10H22N2O4S |
| Molecular Weight | 266.36 |
| Storage Details | Ambient |
| Harmonized Tariff Code | 2933599090 |
Product Specification
| Appearance | White crystalline powder |
| Assay | ≥ 99% |
| Storage Temp | Store at RT |
| Melting point | 211-216°C |
| Acidity coefficient (pKa) | 8.3(at 25℃) |
We understand the importance of protecting the integrity of your biomolecules and reagents with the right buffering systems. Our biological buffers provide solution stability and pH control without interfering with biological processes, and supply critical salts and nutrients for cells and tissues. Our advanced buffering systems can bring you phenomenal stability in cell culture, polymerase chain reaction (PCR), drug screening, bioprocessing, purification, and final formulation applications. All of our products can be scaled from early research to commercial applications in varying buffer grades. We offer numerous packaging configurations , custom blending, and even liquid formulation.
We also supply a comprehensive selection of high-quality biochemicals in a practical range of grades and in a variety of innovative, user-friendly packaging options. In addition to off-the-shelf products, we offer custom reagent manufacturing, blending and packaging to meet your needs.
The working principle and pH value of buffer solution When a certain amount of acid and alkali is added to some solutions, it has the effect of hindering the pH change of the solution, which is called buffering effect. Such a solution is called a buffer solution. Mixed solutions of weak acids and their salts (such as HAc and NaAc), and mixed solutions of weak bases and their salts (such as NH3·H2O and NH4Cl) are buffer solutions. The buffering effect of the buffer solution composed of the weak acid HA and its salt NaA on the acid is due to the presence of a sufficient amount of alkali A- in the solution. When a certain amount of strong acid is added to this solution, H ions are basically consumed by A- ions: so the pH of the solution is almost unchanged; when a certain amount of strong base is added, the weak acid HA present in the solution consumes OH- ions hinder the change of pH.