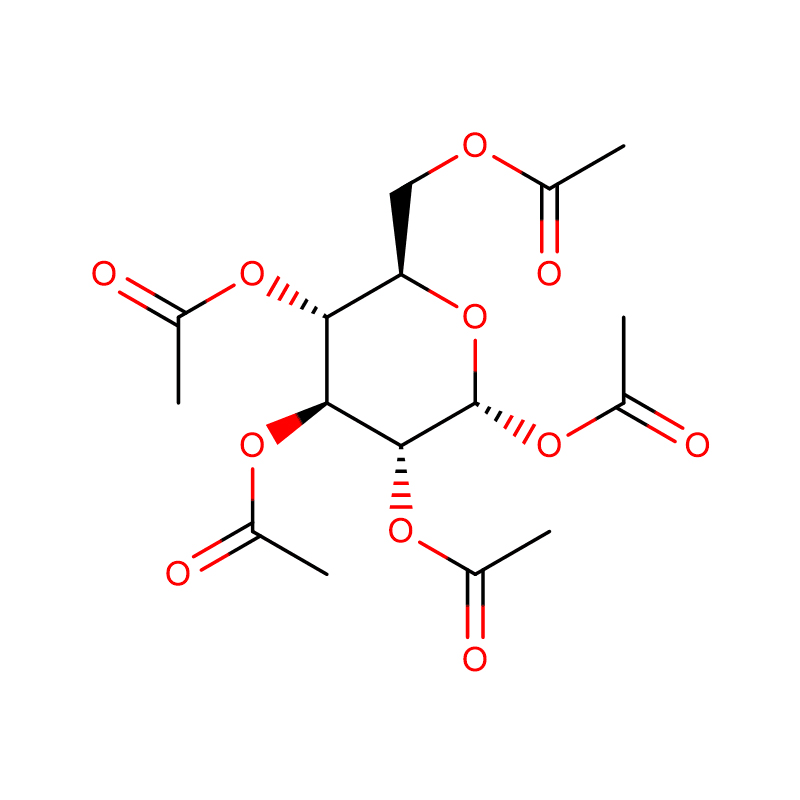
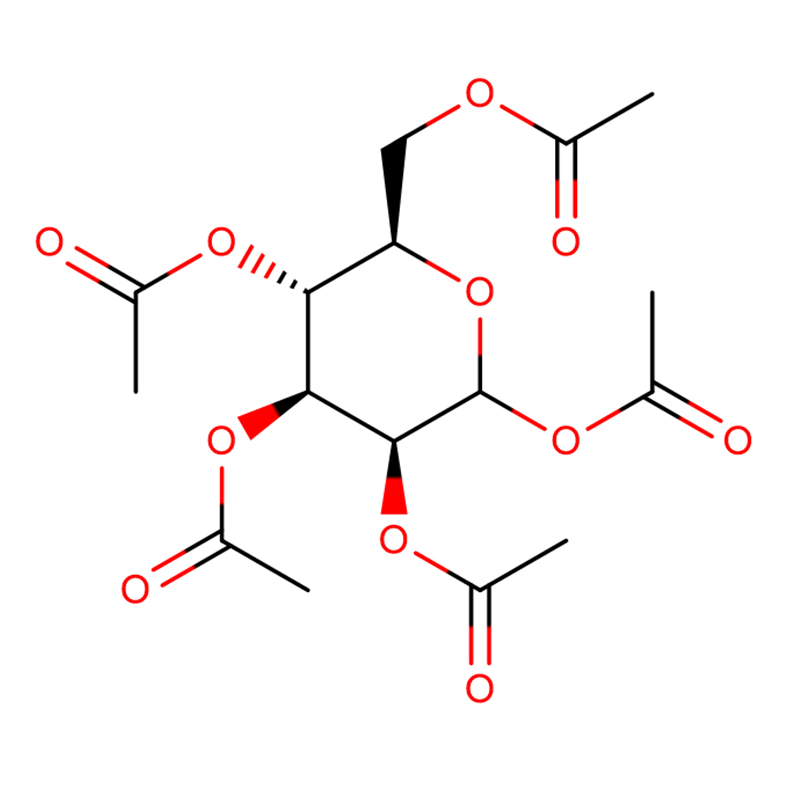
In the presence of 2.8 mM D-glucose, beta-D-glucose pentaacetate (1. 7 mM) augmented insulin release from isolated rat pancreatic islets more than alpha-D-glucose pentaacetate. Likewise, the further increment in insulin output evoked by nateglinide (0.01 mM) was higher in islets exposed to beta- rather than alpha-D-glucose pentaacetate. Inversely, in the presence of 2.8 mM unesterified D-glucose, alpha-L-glucose pentaacetate, but not beta-L-glucose pentaacetate, significantly augmented insulin output. The higher insulinotropic potency of the beta-anomer of D-glucose pentaacetate coincided with the fact that it significantly increased the paired ratio between D-[U-14C]glucose oxidation and D-[5-3H]glucose utilization, whereas alpha-D-glucose pentaacetate failed to do so. These findings are interpreted to support the concept that the stimulation of insulin release by these esters is largely attributable to their direct interaction with a stereospecific receptor, with preference for the configuration of the C1 common to beta-D-glucose pentaacetate and alpha-L-glucose pentaacetate.