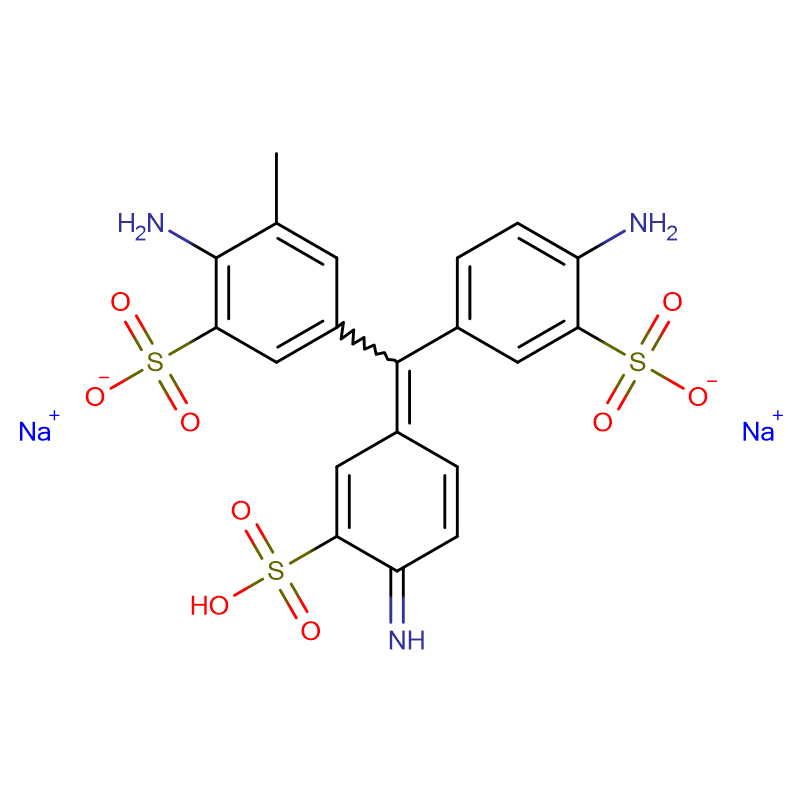
Fuchsin acid CAS:3244-88-0
| Catalog Number | XD90488 |
| Product Name | Fuchsin acid |
| CAS | 3244-88-0 |
| Molecular Formula | C20H20N2O9S3 |
| Molecular Weight | 585.5382 |
| Storage Details | Ambient |
| Harmonized Tariff Code | 32129000 |
Product Specification
| Appearance | dark green crystalline powder |
| Assay | 70% |
| Water Content | 10.0% max |
| Solubility | Clear solution, no particles |
| Strength | 100% min |
| Water insoluble | 0.2% max |
Islet amyloid polypeptide (IAPP; also known as amylin) is responsible for islet amyloid formation in type 2 diabetes, and IAPP-induced toxicity is believed to contribute to the loss of β-cell mass associated with the late stages of type 2 diabetes. Islet amyloid formation may also play a role in graft failure after transplantation. IAPP is produced as a prohormone, pro-islet amyloid polypeptide (proIAPP), and processed in the secretory granules of the pancreatic β-cells. Partially processed forms of proIAPP are found in amyloid deposits; most notable is a 48-residue intermediate, proIAPP(1-48), which includes the N-terminal pro-extension, but which has been properly processed at the C-terminus. Incomplete processing may play a role in islet amyloid formation by promoting interactions with sulfated proteoglycans of the extracellular matrix, which, in turn, promote amyloid formation. We show that acid fuchsin (3-(1-(4-amino-3-methyl-5-sulphonatophenyl)-1-(4-amino-3-sulphonatophenyl)methylene)cyclohexa-1,4-dienesulphonic acid), a simple sulfonated triphenyl methyl derivative, is a potent inhibitor of amyloid formation by the proIAPP(1-48) intermediate. The more complicated triphenyl methane derivative fast green FCF {ethyl-[4-[[4-[ethyl-[(3-sulfophenyl)methyl]amino]phenyl]-(4-hydroxy-2-sulfophenyl)methylidene]-1-cyclohexa-2,5-dienylidene]-[(3-sulfophenyl)methyl]azanium} also inhibits amyloid formation by IAPP and the proIAPP processing intermediate. Both compounds inhibit amyloid formation by mixtures of the proIAPP intermediate and the model glycosaminoglycan heparan sulfate. Acid fuchsin also inhibits glycosaminoglycan-mediated amyloid formation by mature IAPP. The ability to inhibit amyloid formation is not simply due to the compounds being sulfonated, since the sulfonated inhibitor of amyloid-β, tramiprosate, is not an inhibitor of amyloid formation by proIAPP(1-48).