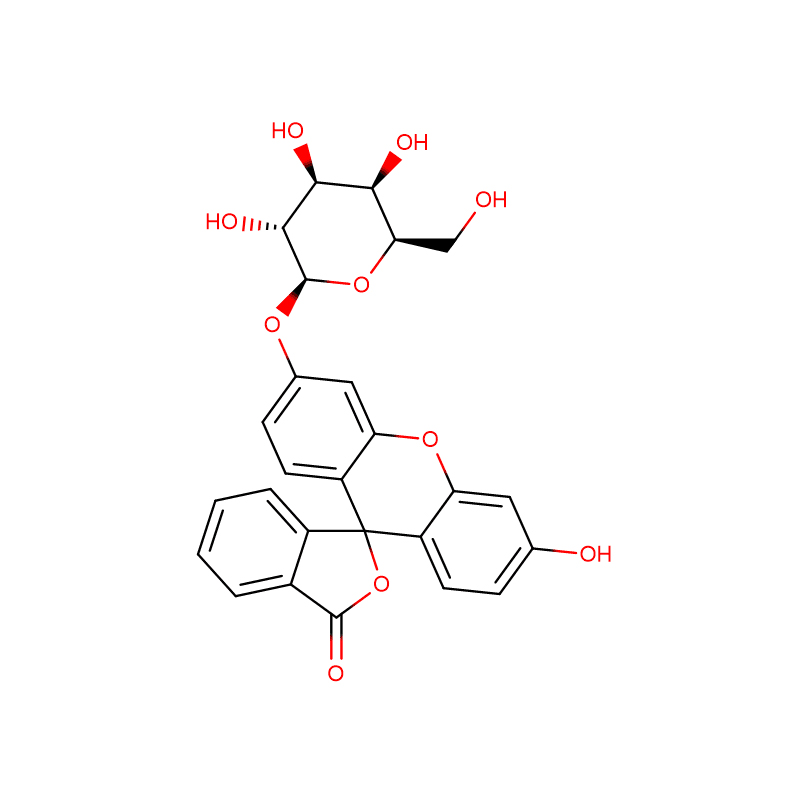
FLUORESCEIN MONO-BETA-D-GALACTOPYRANOSIDE Cas:102286-67-9 99% White powder
| Catalog Number | XD90047 |
| Product Name | FLUORESCEIN MONO-BETA-D-GALACTOPYRANOSIDE |
| CAS | 102286-67-9 |
| Molecular Formula | C26H22O10 |
| Molecular Weight | 494.12 |
| Storage Details | Ambient |
Product Specification
| Appearance | White powder |
| Assay | 99% |
| Density | 1.69g/cm3 |
| Boiling Point | 813.4ºC at 760mmHg |
| Flash Point | 281ºC |
| Refractive Index | 1.772 |
| Storage Conditions | -20ºC |
| Vapor Pressure | 5.73E-28mmHg at 25°C |
Monogalactopyranosides of fluorescein and fluorescein methyl ester: synthesis, enzymatic hydrolysis by biotnylated β-galactosidase, and determination of translational diffusion coefficient
Fluorescein monoglycosides (d-galactopyranoside (FMG) and d-glucopyranoside) and their methyl ester (MFMG) have been prepared from acetobromoglucose/galactose and fluorescein methyl ester in good yields. Enzymatic hydrolysis experiments (using biotinylated β-galactosidase) of the galacto derivatives have been performed and kinetic parameters were calculated. A 15–20 times increase of the fluorescence intensity has been observed during the hydrolysis. A linear increase of fluorescence has been noted at short time and low concentration of substrate, making these compounds useful and sensitive probes for galactosidases. The magnitude of the Michaelis–Menten constant (Km) value for MFMG is higher than that of FMG suggesting a possible conformational change of the fluorogenic substrate. Km value for biotinylated β-Gal with FMG is lower than that for the native enzyme. This observation indicates higher substrate affinity of the biotinylated enzyme in comparison to the native enzyme. Translational diffusion coefficients have been measured, for both fluorogenic substrates and both the products, employing fluorescence correlation spectroscopy. Translational diffusion coefficients for fluorogenic substrates and the enzymatic hydrolysis products have been measured to be similar, in the range of 3.5–4.5 × 10−10 m2 s−1. Thus an enhancement or retardation of the enzymatic kinetics due to difference in translational mobility of substrate and product is not that apparent.