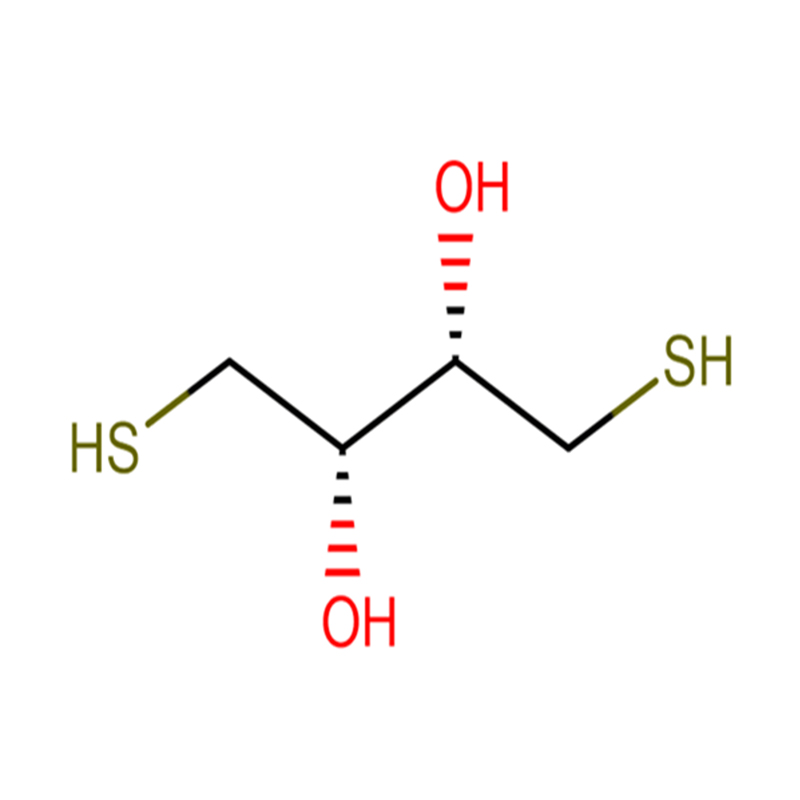
DDT CAS:3483-12-3 >99% White free flowing powder DL-Dithiothreitol
| Catalog Number | XD90007 |
| Product Name | DTT (Dithiothreitol) |
| CAS | 3483-12-3 |
| Molecular Formula | C4H10O2S2 |
| Molecular Weight | 154.25 |
| Storage Details | 2 to 8 °C |
| Harmonized Tariff Code | 29309098 |
Product Specification
| pH | 4 - 6 |
| Loss on Drying | <0.5% |
| Solubility | Soluble in methanol and methylene chloride, water, absolute ethanol, acetone, ethyl acetate |
| Assay | >99% |
| UV Absorbance | @ 500nm: <0.05, @ 280nm: <0.10 |
| Clarity | A) 5% (W/V) solution in water is clear and colourless. B) 1 molar solution in 0.01m sodium acetate at pH 5.2 should be clear and colourless |
| Appearance | White free flowing powder |
| Melting Range | 41 +/- 3 Deg C |
| Related Substance | <0.4% |
| For research use only, not for human use | research use only, not for human use |
Dithiothreitol (DTT), a new type of green additive
Dithiothreitol (DTT), CAS: 3483-12-3, as a widely used scientific research reagent, is often used as a reducing agent for sulfhydryl DNA, a deprotecting agent, and the reduction of disulfide bonds in proteins. A new type of green additive plays an important role in improving battery performance.
Dithiothreitol (DTT) is a strong reducing agent, and its reducibility is largely due to the conformational stability of the six-membered ring (containing disulfide bonds) in its oxidation state. The reduction of a typical disulfide bond by dithiothreitol consists of two consecutive sulfhydryl-disulfide bond exchange reactions. The reducing power of dithiothreitol (DTT) is affected by the pH value, and it can only play a reducing effect when the pH value is greater than 7. This is because only the deprotonated thiolate anions are reactive, while mercaptans do not, and the pKa of mercapto groups is generally 8.3.
Dithiothreitol (DTT) is commonly used to reduce the disulfide bonds of protein molecules and polypeptides. It is usually used as a protein sulfhydryl protective agent and used in vaccine preparations to prevent protein cysteine residues from forming intramolecular and intermolecular disulfides. key. In the process of nucleic acid detection, dithiothreitol (DTT) can destroy the disulfide bonds in the RNase protein, denature the RNase, and facilitate the conduct of experiments such as RNA library building and RNA amplification. Dithiothreitol (DTT) is also used as an antidote to protect cells and tissues, as a radioprotectant, etc.
However, dithiothreitol (DTT) is often unable to reduce the disulfide bonds embedded in the protein structure (solvent inaccessible). The reduction of such disulfide bonds often requires denaturation of the protein first.
In order to inhibit the shuttle effect of lithium-sulfur batteries and improve the electrochemical performance of lithium-sulfur batteries, try to use dithiothreitol (DTT) as a shearing agent to shear high-order polysulfides to prevent them from dissolving. Threitol (DTT) is mixed into multi-walled carbon nanotubes (MWCNTs) paper to prepare a DTT interlayer. The DTT interlayer is placed between the positive electrode sheet and the separator of the lithium-sulfur button half-cell, and the sulfur-carrying surface density of the positive electrode sheet About 2mg/cm2. SEM observation results confirm that DTT is uniformly dispersed on the surface and voids of the MWCNTs paper. Electrochemical test results show that the lithium-sulfur battery with DTT sandwich structure has a first discharge specific capacity of 1288 mAh/g at a rate of 0.05C. For the first time, the coulombic efficiency is close to 100%, and the specific capacity during charge and discharge at 0.5C, 2C, and 4C rates reaches 650mAh/g, 600mAh/g, and 410mAh/g, respectively. The introduction of the DTT sandwich structure can effectively shear high-order polysulfides. It prevents it from migrating to the lithium negative electrode, thereby inhibiting the shuttle effect and improving the cycle stability and coulomb efficiency of lithium-sulfur batteries.
It is worth noting that dithiothreitol (DTT) is a toxic substance. For example, in the presence of transition metals, dithiothreitol (DTT) can cause oxidative damage to biological molecules. At the same time, dithiothreitol (DTT) ) Can also enhance the toxicity of some compounds containing arsenic and mercury. Dithiothreitol (DTT) has a pungent odor, which can be harmful to health due to inhalation and skin contact. Therefore, it is necessary to protect it during operation, wear masks, gloves and goggles, and operate in a fume hood.
Thithreitol (DTT) as a shearing agent in lithium-sulfur batteries
Lithium-sulfur battery is considered to be a battery system with great potential due to its high energy density and environmental protection. However, the "shuttle effect" of polysulfides leads to poor cycle life and serious self-discharge, which restricts its application. reason.
Thiothreitol (DTT) can be added to the battery as a shearing agent. It can rapidly shear disulfide bonds at room temperature, shear high-order polysulfides to prevent their dissolution, inhibit the shuttle effect, and increase lithium The electrochemical performance of sulfur batteries.
Dithiothreitol (DTT) as an electrolyte additive in alkaline aluminum/air batteries
In alkaline aluminum/air batteries, dithiothreitol can form a uniform and stable protective layer through dynamic covalent bonds on the surface of the aluminum anode, inhibit the self-corrosion of the aluminum anode, and effectively improve its performance.