China Manufacturer for Piperazine-1,4-Bis(2-Ethanesulfonic Acid) Disodium Salt - Vancomycin hydrochloride Cas: 1404-93-9 White almost white or tan to pink powder – XD BIOCHEM
China Manufacturer for Piperazine-1,4-Bis(2-Ethanesulfonic Acid) Disodium Salt - Vancomycin hydrochloride Cas: 1404-93-9 White almost white or tan to pink powder – XD BIOCHEM Detail:
| Catalog Number | XD90197 |
| Product Name | Vancomycin hydrochloride |
|
CAS |
1404-93-9 |
|
Molecular Formula |
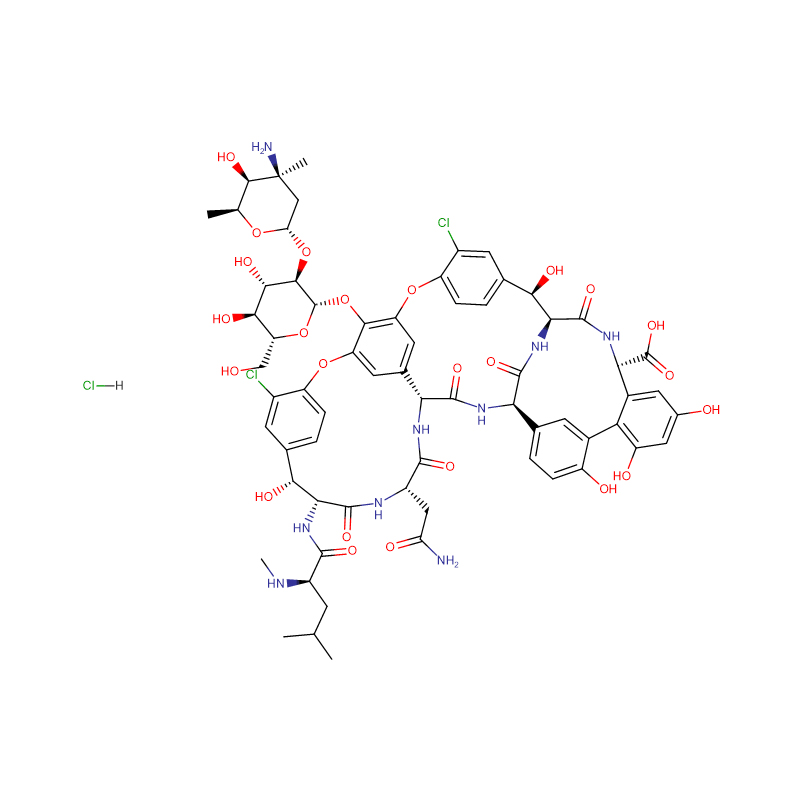
C66H76Cl3N9O24 |
|
Molecular Weight |
1485.7145 |
| Storage Details | 2 to 8 °C |
| Harmonized Tariff Code | 29419000 |
Product Specification
| Water | NMT 5.0% |
| Heavy metals | NMT 30ppm |
| pH | 2.5 – 4.5 |
| Bacterial endotoxins | NMT 0.33EU/mg of Vancomycin |
| Clarity of Solution | Clear |
| Appearance | White, almost white, or tan to pink powder |
| Vancomycin B | NLT 85% |
| Limit of monodechlorovancomycin |
NMT 4.7% |
| Assay (microbial, anhydrous basis) |
NLT 900ug/mg |
1.The incidence of community-acquired methicillin-resistant Staphylococcus aureus infections is rising at an alarming pace. Effective treatment has historically involved early débridement and antibiotic administration. This study was designed to prospectively determine the effectiveness of empiric therapy in treating hand infections.A prospective randomized trial was conducted at a level I county hospital. Patients with a hand infection received either empiric intravenous vancomycin at admission or intravenous cefazolin. Outcomes were tracked using severity of infection, appropriate clinical response, and length of stay. Cost-effectiveness was calculated using total cost for each patient in both groups. Statistical analyses were performed.Forty-six patients were enrolled in the study. Twenty-four were randomized to cefazolin (52.2 percent) and 22 (47.8 percent) to vancomycin. There was no statistical difference between cost of treatment (p < 0.20) or mean length of stay (p < 0.18) betw een the groups. Patients randomized to cefazolin had higher mean costs of treatment compared with patients who were randomized to vancomycin (p < 0.05). Patients with more severe infections had more expensive mean costs of treatment (p < 0.0001) and longer mean length of stay (p = 0.0002). Near the end of the study, the incidence of community-acquired methicillin-resistant S. aureus at the authors’ county hospital was discovered to be 72 percent, which caused the study to be terminated prematurely by the institutional review board because of the high incidence precluding further randomization.Appropriate early treatment for methicillin-resistant S. aureus has not been definitively established. No difference in outcome using cefazolin versus vancomycin as a first-line agent was identified.
2.With the improvements in wound healing through the use of intravenous prophylactic antibiotics and technical refinements, postoperative elbow infections have become less common but still occur in certain elective elbow surgeries. The objective of this study was to evaluate the safety and efficacy of prophylactic application of vancomycin into the operative site to reduce the incidence of infection after the open release of post-traumatic stiff elbows.A retrospective review of 272 such patients during a 4-year period was performed. In the control group (93 patients), simple prophylaxis with standard intravenous antibiotics was performed; in the vancomycin group (179 patients), vancomycin powder was applied directly into the wound before closure along with standard intravenous prophylaxis.After a follow-up of at least 6 months, the control group was found to have 6 infections (6.45%; confidence interval: 2.40%-13.52%) compared with none (0%; confidence interval: 0-2%.04%) in the vancom ycin group, which was a statistically significant difference (P = .0027). No adverse effects were documented from the direct use of the vancomycin powder.The local application of vancomycin powder may be a promising means of preventing postoperative elbow infections after elbow release in patients with post-traumatic elbow stiffness.
Product detail pictures:
Related Product Guide:
"Based on domestic market and expand overseas business" is our improvement strategy for China Manufacturer for Piperazine-1,4-Bis(2-Ethanesulfonic Acid) Disodium Salt - Vancomycin hydrochloride Cas: 1404-93-9 White almost white or tan to pink powder – XD BIOCHEM , The product will supply to all over the world, such as: Lithuania, Mexico, Swiss, Our company sets up several departments, including production department, sales department, quality control department and sevice center,etc. only for accomplish the good-quality product to meet customer's demand, all of our products have been strictly inspected before shipment. We always think about the question on the side of the customers,because you win,we win!
In general, we are satisfied with all aspects, cheap, high-quality, fast delivery and good procuct style, we will have follow-up cooperation!