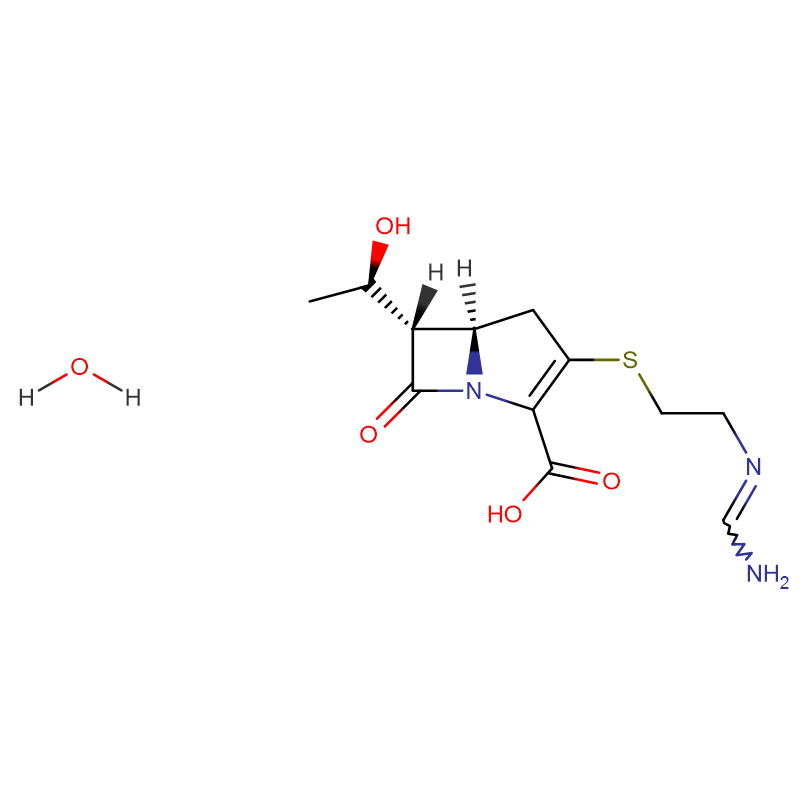
Ceftazidime pentahydrate Cas: 78439-06-2
| Appearance | White or almost white crystalline powder |
| Assay | 99% min |
| Water | 13-15% |
| Heavy metals | 20ppm max |
| Identification | Complies |
| pH | 3.0-4.0 |
| Residue on Ignition | 0.2% max |
| Pyridine | ≤0.05% |
| Bacterial endotoxins | ≤0.1 Eu/mg |
| Transmittance | ≥90% |
Product Specification
| Appearance | White or almost white crystalline powder |
| Assay | 99% min |
| Water | 13-15% |
| Heavy metals | 20ppm max |
| Identification | Complies |
| pH | 3.0-4.0 |
| Residue on Ignition | 0.2% max |
| Pyridine | ≤0.05% |
| Bacterial endotoxins | ≤0.1 Eu/mg |
| Transmittance | ≥90% |
The stability of ceftazidime to beta lactamase is better. The probability of resistance to drug resistance is low and the side effects are less. The third generation of broad-spectrum cephalosporins are stable to a variety of lactamases, and have strong bactericidal effect on Gram-positive and negative bacteria and anaerobic strains, and the only effective and unique to Pseudomonas aeruginosa is the only one. Cephalosporins, which can replace aminoglycosides, are called the fourth generation cephalosporins. Severe infections caused by sensitive bacteria (such as septicemia, meningitis, bacteremia, etc.), respiratory infection (such as pneumonia, bronchitis, etc.), ear nose and throat infection, skin and soft tissue infection, urinary tract infection, gastrointestinal, biliary and abdominal infection, bone and joint infection, etc.