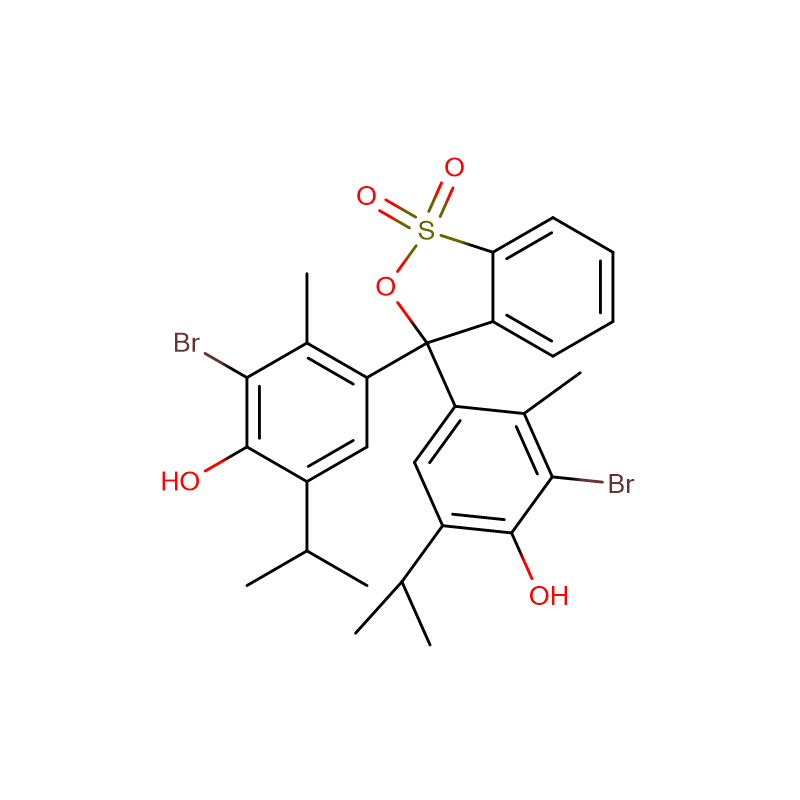
Bromothymol blue, free acid Cas: 76-59-5 Purple/ brown powder
| Catalog Number | XD90526 |
| Product Name | Bromothymol blue, free acid |
|
CAS |
76-59-5 |
|
Molecular Formula |
C27H28Br2O5S |
|
Molecular Weight |
624.38 |
| Storage Details | Ambient |
| Harmonized Tariff Code | 29349990 |
Product Specification
|
Appearance |
Purple/ brown powder |
|
Assay |
99% |
|
Loss on Drying |
3% max |
|
Dye Content |
95% min |
|
Transition Range |
pH 5.8 - 7.6 Yellow - Blue |
|
Solubility at 0.1% (95% Ethanol) |
Clear Solution |
|
Wavelength of maximum absorption(pH 5.8) λ1 max |
430 - 435 nm |
|
Wavelength of maximum absorption (pH 7.6) λ2 max |
615 - 618 nm |
|
Absorptivity (E 1% in 1cm cell at λ1 max),pH 5.8 |
260 - 300 |
|
Absorptivity (E 1% in 1cm cell at λ2 max),pH 7.6 |
470 - 520 |
The bioelectrochemical behavior of three triphenylmethane (TPM) dyes commonly used as pH indicators, and their application in mediated electron transfer systems for glucose oxidase bioanodes in biofuel cells was investigated. Bromophenol Blue, Bromothymol Blue, Bromocresol Green were compared bioelectrochemically against two widely used mediators, benzoquinone and ferrocene carboxy aldehyde. Biochemical studies were performed in terms of enzymatic oxidation, enzyme affinity, catalytic efficiency and co-factor regeneration. The different features of the TPM dyes as mediators are determined by the characteristics in the oxidation/reduction processes studied electrochemically. The reversibility of the oxidation/reduction processes was also established through the dependence of the voltammetric peaks with the sweep rates. All three dyes showed good performances compared to the FA and BQ when evaluated in a half enzymatic fuel cell. Potentiodynamic and power response experiments showed maxima power densities of 32.8 μW cm −2 for ferrocene carboxy aldehyde followed by similar values obtained for TPM dyes around 30 μW cm −2 using glucose and mediator concentrations of 10 mmol L −1 and 1.0 mmol L −1, respectively. Since no mediator consumption was observed during the bioelectrochemical process, and also good redox re-cycled processes were achieved, the use of triphenylmethane dyes is considered to be promising compared to other mediated systems used with glucose oxidase bioanodes and/or biofuel cells.