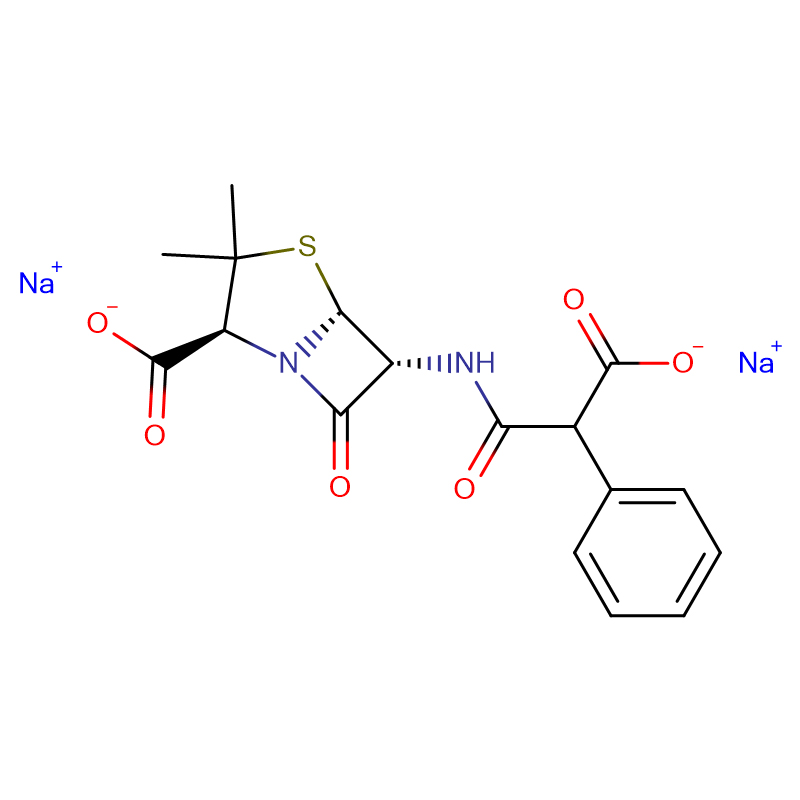
Carbenicillin disodium salt CAS:4800-94-6 White to off-white powder
| Catalog Number | XD90371 |
| Product Name | Carbenicillin disodium salt |
| CAS | 4800-94-6 |
| Molecular Formula | C17H16N2Na2O6S |
| Molecular Weight | 422.36 |
| Storage Details | 2 to 8 °C |
| Harmonized Tariff Code | 29411000 |
Product Specification
| pH | 5.5-7.5 |
| Water Content | ≤ 6.0% |
| Solubility | Clear and slightly yellow solution |
| Assay | 99% |
| Potency | 830ug/mg |
| Pyrogens | ≤ 80mg/kg |
| Transmittance | Complies |
| Appearance | White to off-white powder |
| Iodine Absorbing Substances | ≤ 8.0% |
| Usp grade | Complies |
| Assay (penicillin G) | Complies |
Cystic fibrosis is a genetic disorder in which abnormal mucus in the lungs is associated with susceptibility to persistent infection. Pulmonary exacerbations are when symptoms of infection become more severe. Antibiotics are an essential part of treatment for exacerbations and inhaled antibiotics may be used alone or in conjunction with oral antibiotics for milder exacerbations or with intravenous antibiotics for more severe infections. Inhaled antibiotics do not cause the same adverse effects as intravenous antibiotics and may prove an alternative in people with poor access to their veins.To determine if treatment of pulmonary exacerbations with inhaled antibiotics in people with cystic fibrosis improves their quality of life, reduces time off school or work and improves their long-term survival.We searched ClinicalTrials.gov and the Australia and New Zealand Clinical Trials Registry for relevant trials. Date of last search: 15 March 2012We also searched the Cochrane Cystic Fibrosis G roup's Cystic Fibrosis Trials Register. Date of the last search: 01 June 2012.Randomised controlled trials in people with cystic fibrosis with a pulmonary exacerbation in whom treatment with inhaled antibiotics was compared to placebo, standard treatment or another inhaled antibiotic for between one and four weeks.Two review authors independently selected eligible trials, assessed the risk of bias in each trial and extracted data. Authors of the included trials were contacted for more information.Six trials with 208 participants were included in the review. Trials were heterogenous in design and interventions (however, all included trials compared inhaled versus intravenous antibiotic regimens). Risk of bias was difficult to assess in most trials. Results were not fully reported and only limited data were available for analysis. Four trials reported some results on forced expiratory volume at one second and found no significant differences between the inhaled antibiotic and the compari son intervention. In two of these trials using 300 mg of inhaled tobramycin, the change in forced expiratory volume at one second was similar to intravenous tobramycin; and in one trial the time until the next exacerbation was not different. No important adverse effects were reported.There is little useful high-level evidence to judge the effectiveness of inhaled antibiotics for the treatment of pulmonary exacerbations in people with cystic fibrosis. The included trials were not sufficiently powered to achieve their goals. Hence, we are unable to demonstrate whether one treatment was superior to the other or not. Further research is needed to establish whether inhaled tobramycin may be used as an alternative to intravenous tobramycin for some pulmonary exacerbations.