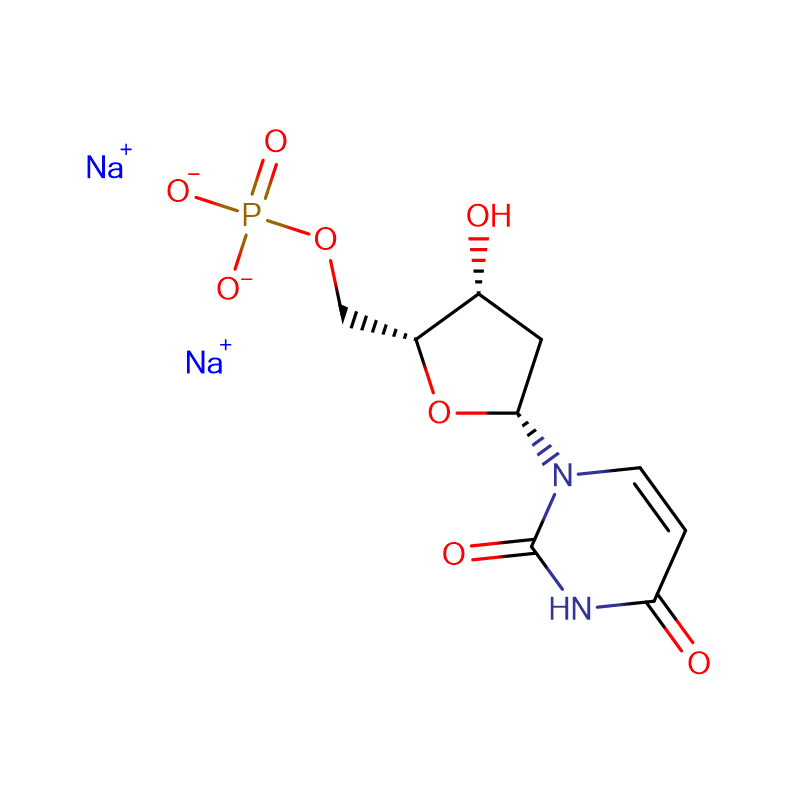
Adenosine 5′-monophosphate disodium salt Cas:4578-31-8
| Catalog Number | XD90587 |
| Product Name | Adenosine 5'-monophosphate disodium salt |
|
CAS |
4578-31-8 |
|
Molecular Formula |
C10H12N5Na2O7P |
|
Molecular Weight |
391.18 |
| Storage Details | Ambient |
| Harmonized Tariff Code | 29349990 |
Product Specification
| Appearance | White or off white powder |
| Assay | 99% |
| Heavy metals | <10ppm |
| Storage Temperature | +20 ° C |
| Water Content | <26.0% |
Monitoring of pharmacodynamics in addition to pharmacokinetics is one of strategies to individualize mycophenolate mofetil therapy. The purpose of this study was to develop sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods for evaluation of the pharmacokinetics and pharmacodynamics of mycophenolic acid (MPA). Concentrations of mycophenolic acid glucuronide (MPAG), mycophenolic acid acyl-glucuronide, as well as unbound MPA and MPAG, were determined, and inosine-5'-monophosphate dehydrogenase activity was calculated by measuring concentrations of produced xanthosine-5'-monophosphate (XMP) and intracellular adenosine-5'-monophosphate after incubation of peripheral blood mononuclear cell (PBMC) lysates. A metal-free Mastro(TM) column and two gradient patterns were used to improve the quantification limit of XMP to 0.1 μM. In the clinical MPA concentration range, the linearity of the calibration curve, inter- and intra-day precision and accuracy satisfied the relev ant US Food and Drug Administration guidelines. The MPA concentrations in hematopoietic stem cell transplant (HSCT) patients determined by the enzyme assay and the present LC-MS/MS method showed a good correlation (r(2) = 0.95, p < 0.001). In this study, we report sensitive and validated LC-MS/MS methods to evaluate the pharmacokinetics and pharmacodynamics of MPA, which are sufficiently sensitive to assess small quantities of PBMC lysates collected shortly after HSCT.