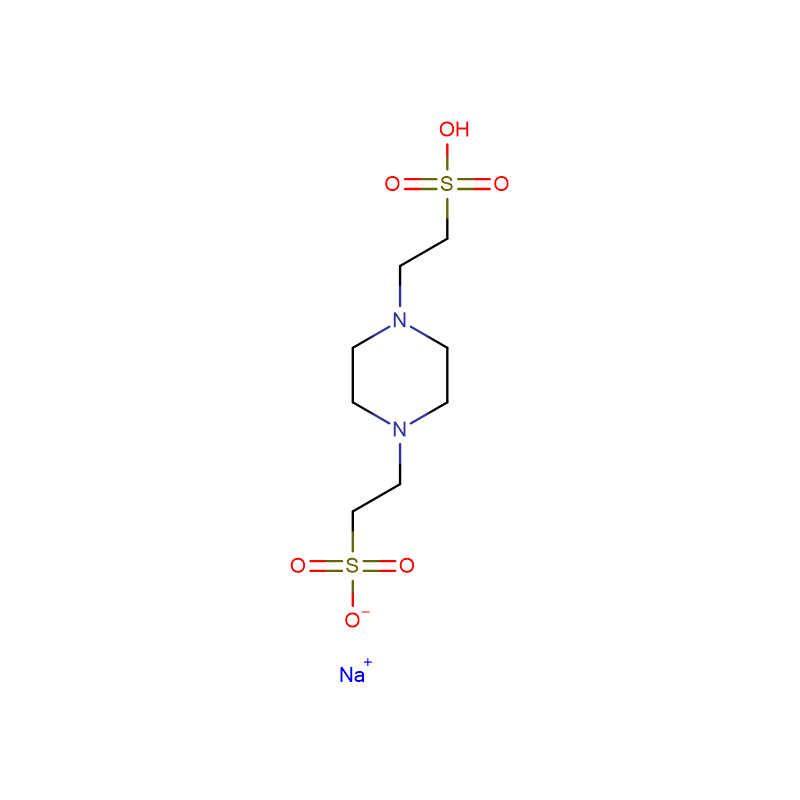
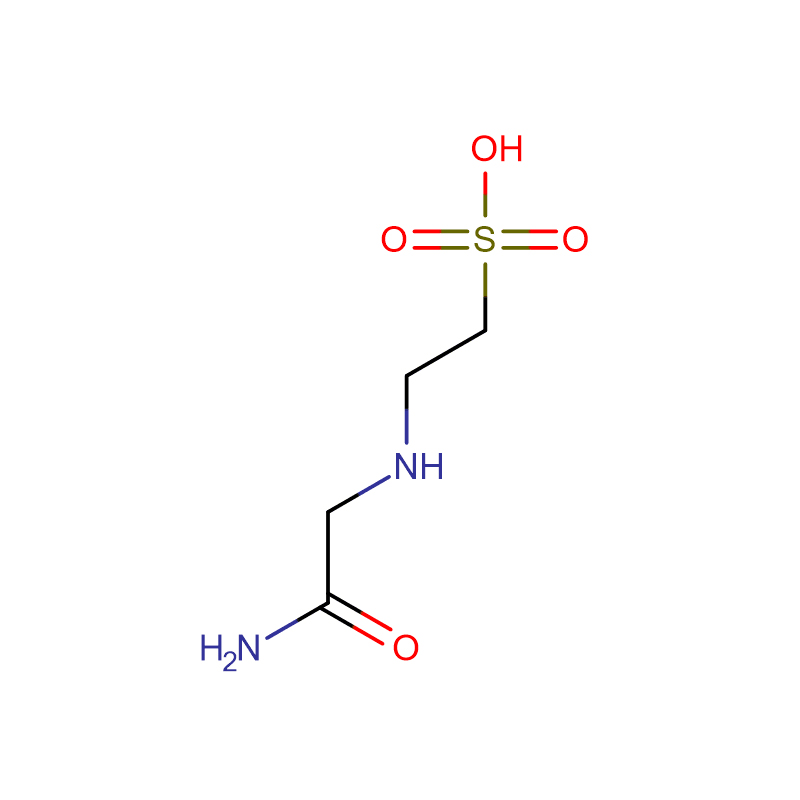
ACES Cas: 7365-82-4 White crystalline powder 99% N-(Carbamoylmethyl)taurine
| Catalog Number | XD90108 |
|
Product Name |
ACES (N-(2-Acetamido)-2-aminoethanesulfonic acid) |
|
CAS |
7365-82-4 |
|
Molecular Formula |
C4H10N2O4S |
|
Molecular Weight |
182.2 |
|
Storage Details |
Ambient |
|
Harmonized Tariff Code |
29241900 |
Product Specification
|
Lead |
<5ppm |
|
pH |
3.5 - 4.5 |
|
Water Content |
<0.5% |
|
Iron |
<5ppm |
|
Residue on Ignition |
<0.1% |
|
Appearance |
White crystalline powder |
|
A260 (0.1M water) |
<0.020 |
|
A280 (0.1M water) |
<0.010 |
| Assay (Titration) on dried basis |
>99% |
The objective of this study was to compare the efficacy of valproate versus haloperidol in decreasing the agitation level in affected patients in the emergency department. We assigned 80 acutely agitated patients to receive either intravenous sodium valproate (20 mg/kg) or intramuscular haloperidol (5 mg/1 ml). Agitation was measured at baseline and 30 min after the first injection using the Agitation-Calmness Evaluation Scale (ACES), the Positive and Negative Syndrome Scale-Excited Component subscale, and the Agitated Behavior Scale. For 80 patients treated with sodium valproate, the mean ± SD dosage was 1541.5 ± 286 mg (range 940-2400). The mean postintervention ACES scores from baseline to 30 min after drug injection were 4.73 (SD = 1.93) for the valproate group and 5.45 (SD = 2.09) for the haloperidol group (P = 0.028). No significant differences were observed in terms of the mean changes 30 min after the intervention for two additional agitation scales. A larger proportion of patients in the haloperidol group experienced intense sedation (36.2%, P < 0.001) and extrapyramidal symptoms (8.7%, P = 0.007) compared with the valproate group (2.5% for intense sedation, no patient for extrapyramidal symptoms). The findings suggest that in the clinical practice setting of emergency psychiatry, intravenous valproate is as effective as haloperidol in reducing agitation, with a better safety profile.


![BES Cas: 10191-18-1 White powder 99% 2-[N,N-Bis(2-hydroxyethyl)amino]ethanesulfonic acid](https://cdn.globalso.com/xdbiochems/10191-18-1.jpg)