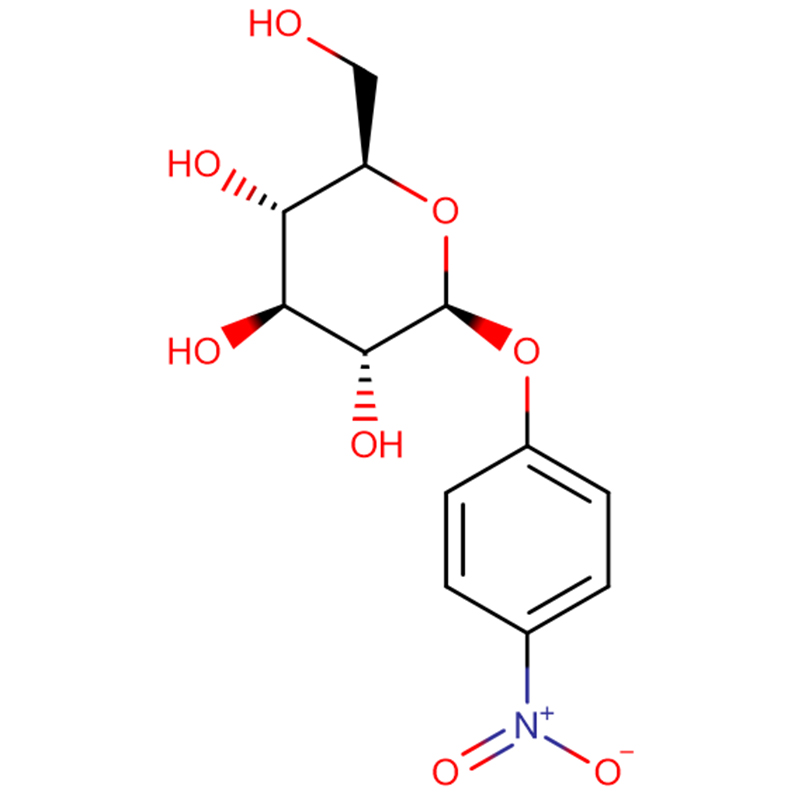
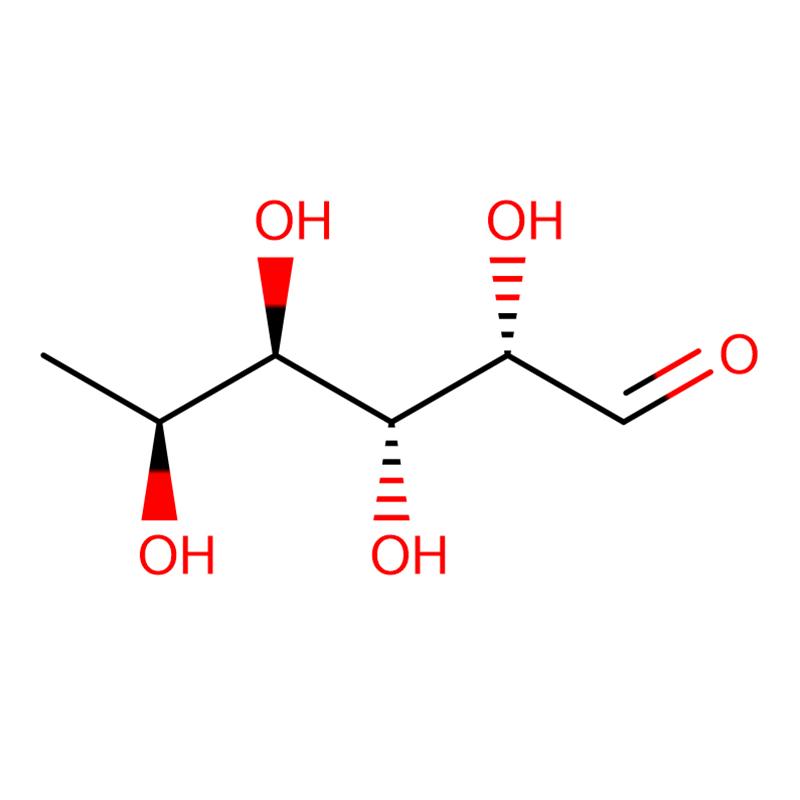
A novel β-glucosidase gene, bgl1G5, was cloned from Phialophora sp. G5 and successfully expressed in Pichia pastoris. Sequence analysis indicated that the gene consists of a 1,431-bp open reading frame encoding a protein of 476 amino acids. The deduced amino acid sequence of bgl1G5 showed a high identity of 85 % with a characterized β-glucosidase from Humicola grisea of glycoside hydrolase family 1. Compared with other fungal counterparts, Bgl1G5 showed similar optimal activity at pH 6.0 and 50 °C and was stable at pH 5.0–9.0. Moreover, Bgl1G5 exhibited good thermostability at 50 °C (6 h half-life) and higher specific activity (54.9 U mg–1). The K m and V max values towards p-nitrophenyl β-D-glucopyranoside (pNPG) were 0.33 mM and 103.1 μmol min–1 mg–1, respectively. The substrate specificity assay showed that Bgl1G5 was highly active against pNPG, weak on p-nitrophenyl β-D-cellobioside (pNPC) and p-nitrophenyl-β-D-galactopyranoside (ONPG), and had no activity on cellobiose.