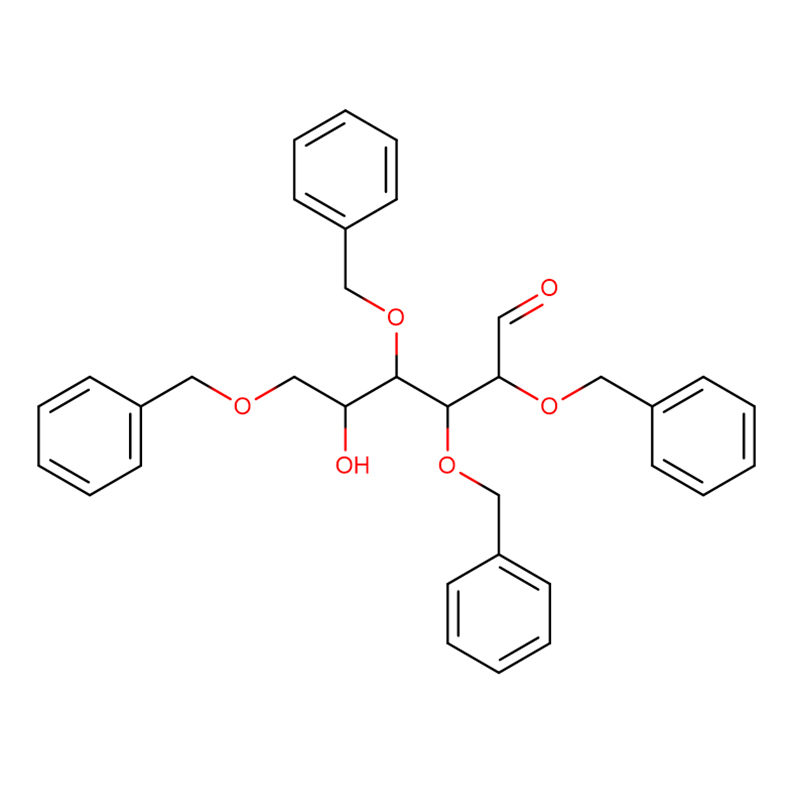
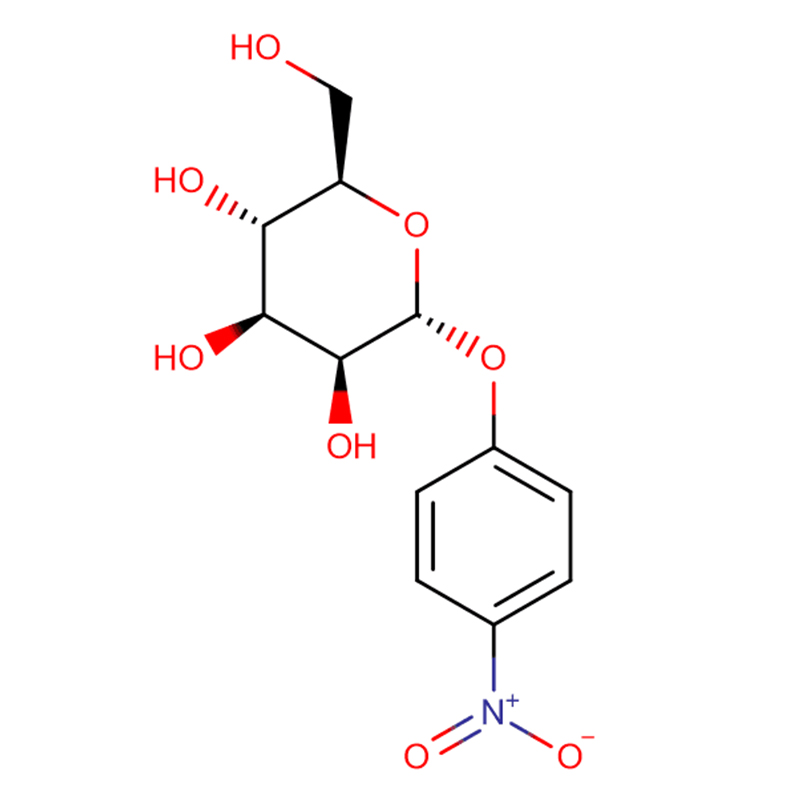
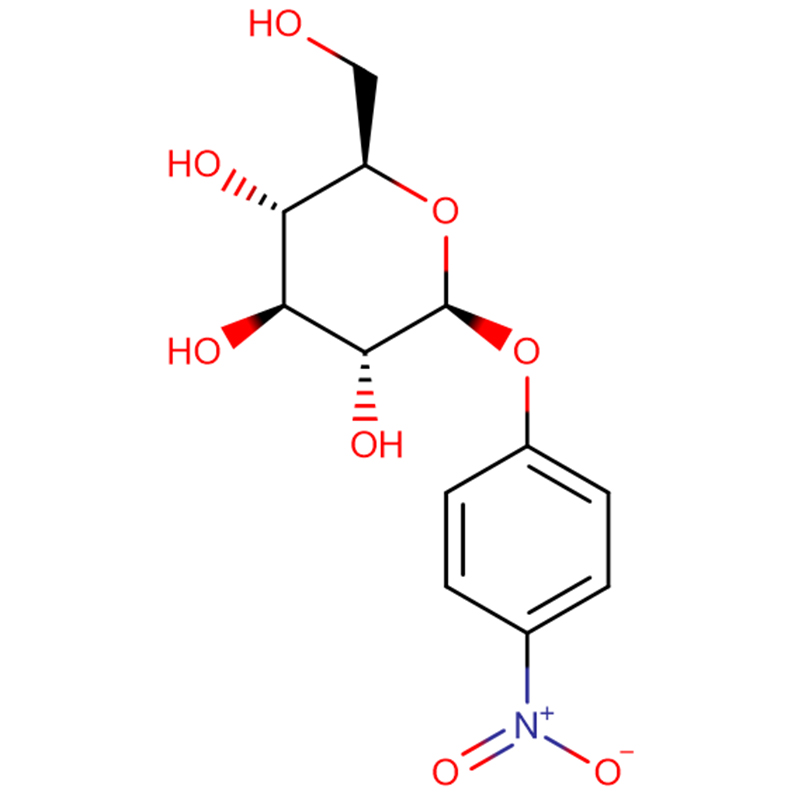
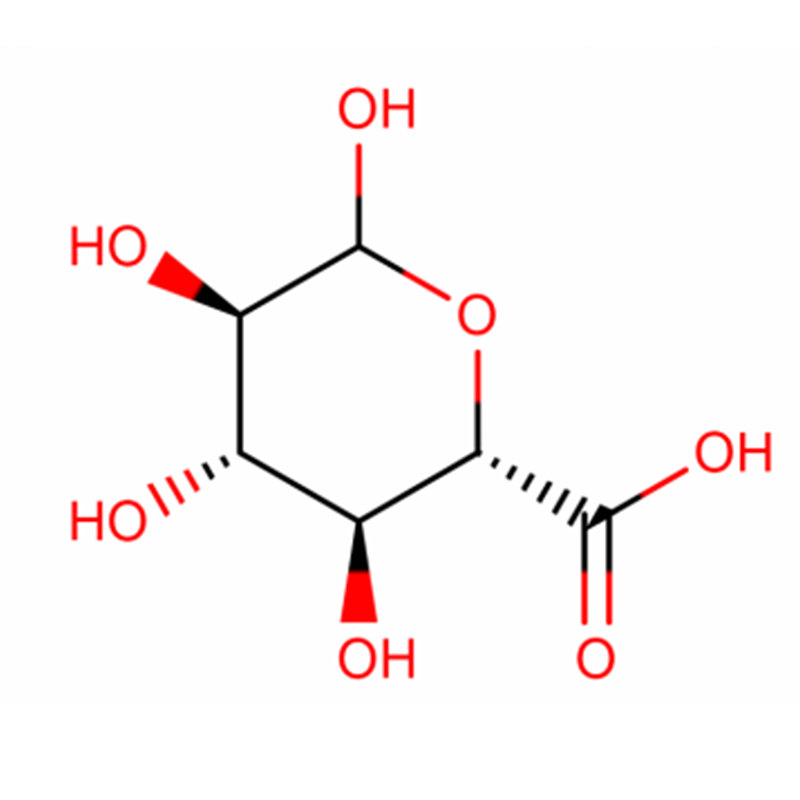
Two isomeric bicyclo[4.1.0]heptane analogues of the glycosidase inhibitor galacto-validamine, (1R*,2S,3S,4S,5S,6S*)-5-amino-1-(hydroxymethyl)bicyclo[4.1.0]heptane-2,3,4-triol, have been synthesized in 13 steps from 2,3,4,6-tetra-O-benzyl-D-galactose. The inhibitory activities of the two conformationally restricted amines, and their corresponding acetamides, were measured against commercial alpha-galactosidase enzymes from coffee bean and E. coli. The activity of the glycosyl hydrolase family GH27 enzyme (coffee bean) was competitively inhibited by the 1R,6S-amine (7), a binding interaction that was characterized by a K(i) value of 0.541 microM. The GH36 E. coli alpha-galactosidase exhibited a much weaker binding interaction with the 1R,6S-amine (IC(50)= 80 microM). The diastereomeric 1S,6R-amine (9) bound weakly to both galactosidases, (coffee bean, IC(50)= 286 microM) and (E. coli, IC(50)= 2.46 mM).