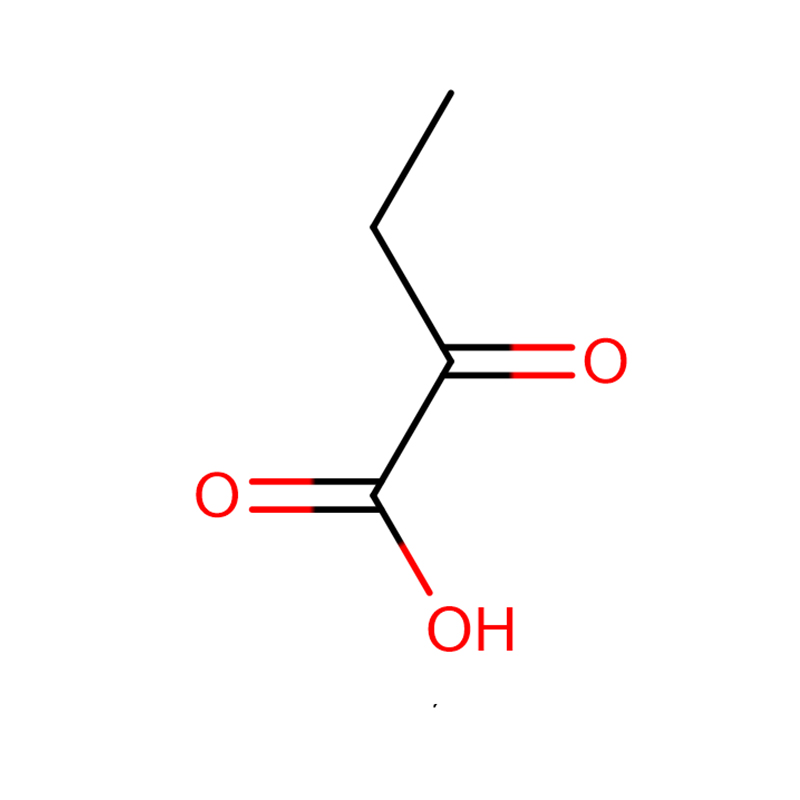
2-Oxobutyric acid CAS:600-18-0 colorless paste
| Catalog Number | XD90263 |
| Product Name | 2-Oxobutyric acid |
| CAS | 600-18-0 |
| Molecular Formula | C4H6O3 |
| Molecular Weight | 102.089 |
| Storage Details | -20 °C |
| Harmonized Tariff Code | 2918300090 |
Product Specification
| Density | 1.182 |
| Melting point | 30-34 °C |
| Boiling point | 84 °C |
| Appearance | colorless paste |
| Assay | 99% |
1.Optically active D-2-hydroxybutanoate is an important building block intermediate for medicines and biodegradable poly(2-hydroxybutanoate). Kinetic resolution of racemic 2-hydroxybutanoate may be a green and desirable alternative for D-2-hydroxybutanoate production. In this work, D-2-hydroxybutanoate at a high concentration (0.197 M) and a high enantiomeric excess (99.1%) was produced by an NAD-independent L-lactate dehydrogenase (L-iLDH) containing biocatalyst. 2-Oxobutanoate, another important intermediate, was co-produced at a high concentration (0.193 M). Using a simple ion exchange process with the macroporous anion exchange resin D301, D-2-hydroxybutanoate was separated from the biotransformation system with a high recovery of 84.7%.
2.Asymmetric synthesis of an unnatural amino acid was demonstrated by omega-transaminase from Vibrio fluvialis JS17. L-2-Aminobutyric acid was synthesized from 2-oxobutyric acid and benzylamine with an enantiomeric excess higher than 99%. The reaction showed severe product inhibition by benzaldehyde, which was overcome by employing a biphasic reaction system to remove the inhibitory product from the aqueous phase. In a typical biphasic reaction (50 mM 2-oxobutyric acid, 70 mM benzylamine and 2.64 U/ml purified enzyme) using hexane as an extractant, conversion of 2-oxobutyric acid reached 96% in 5 h whereas only 39% conversion was obtained without the product extraction.