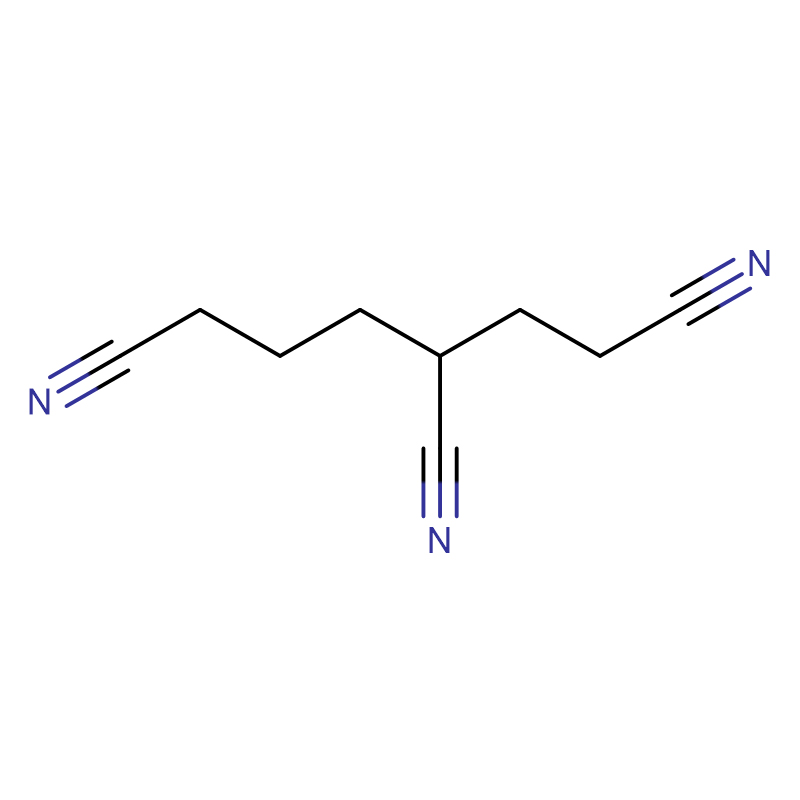
1,3,6-Hexanetricarbonitrile CAS:1772-25-4
| Catalog Number | XD90743 |
| Product Name | 1,3,6-Hexanetricarbonitrile |
| CAS | 1772-25-4 |
| Molecular Formula | C9H11N3 |
| Molecular Weight | 161.204 |
| Storage Details | Ambient |
| Harmonized Tariff Code | 2926909090 |
Product Specification
| Appearance | |
| Assay | 99% |
1,3,6-Hexanetricarbonitrile is an important intermediate for many industrial applications. For example, the tricarboxy group that can be used as a detergent can be obtained by hydrolysis. The corresponding hydrogenation of the trinitrile yields 1,3,6-triaminohexane, which can then be phosgenated in a further step to yield 1,3,6-triisocyanatohexane. This compound is used as an important basic building block in polyurethane (PU) chemistry, for example in the preparation of polyurethane adhesives or polyurethane coatings. 1,3,6-Hexanetrinitrile is an important electrolyte additive, and the composition of the electrolyte limits the application of positive and negative electrode materials in high voltage, traditional organic carbonates (such as chain carbonate DEC, DMC, EMC and cyclic carbonate PC, EC, etc.) will decompose under high voltage [2,3]. Therefore, the development of new organic solvents with wide electrochemical windows, high solubility to lithium salts, and low toxicity has become one of the focuses in the development of high-voltage electrolytes. Nitrile organic solvents usually have excellent properties such as wide electrochemical window, high anode stability, low viscosity and high boiling point [4]. In addition, the decomposition products of organic solvents containing nitrile groups are generally carboxylates, aldehydes or corresponding organic solvents. Amine, no highly toxic CN- ions will be produced during use [5-7]. Nitrile solvents have wide electrochemical windows and are promising new organic solvents. However, from the perspective of the electrochemical performance of lithium-ion batteries, nitrile solvents still have compatibility problems with negative electrodes. Forming a blend system with carbonate solvents or adding blend salt LiBOB can improve this problem to a certain extent.


![Thieno[3,2-b]thiophene Cas:251-41-2 White to Gray to Brown powder to crystal](https://cdn.globalso.com/xdbiochems/251-41-2.jpg)